Abstract
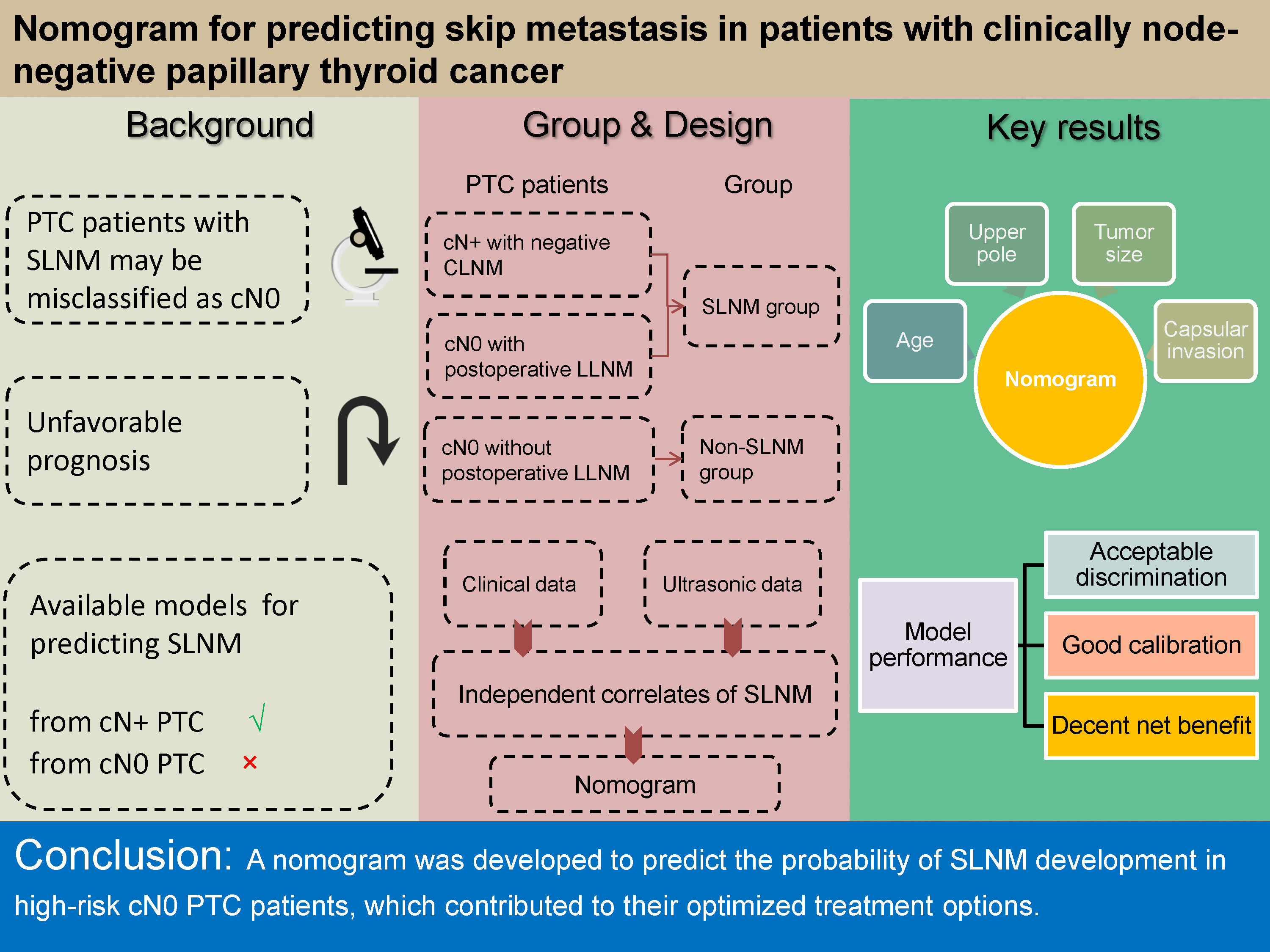
Background. Skip lymph node metastasis (SLNM) refers to lateral lymph node metastasis (LLNM) without involving central lymph node (CLN). Some microscopic nodal positivity may be difficult to detect before surgery due to atypical imaging characteristics. These patients are misdiagnosed as having clinically node-negative (cN0) papillary thyroid cancer (PTC) even after central lymph node dissection, leading to a high risk of developing LNM after surgery. Current prediction models have limited clinical utility, as they are only applicable to predict SLNM from clinically node-positive (cN+) PTC, not cN0 PTC, and this has little impact on treatment strategies.
Objectives. This study aimed to establish a nomogram for preoperatively assessing the likelihood of SLNM in cN0 PTC patients with increased risk of LNM, thus optimizing their therapeutic options.
Materials and methods. The records of 780 PTC patients undergoing thyroidectomy along with bilateral central lymph node dissection were retrospectively reviewed. The cN0 patients with postoperative LLNM (occult SLNM) and cN+ patients without central lymph node metastasis (CLNM) (typical SLNM) were included in the SLNM group (n = 82). The CLNM-negative cN0 patients without postoperative LLNM were assigned to the non-SLNM group (n = 698). The independent correlates of SLNM constituted the nomogram for determining the likelihood of SLNM in high-risk cN0 PTC patients.
Results. The independent correlates of SLNM were age (hazard ratio (HR) = 1.016), tumor location (HR = 1.801), tumor size (HR = 1.528), and capsular invasion (HR = 2.941). They served as components in the development of the nomogram. This model was verified to present acceptable discrimination. It showed good calibration and a decent net benefit when the predicted probability was <60%.
Conclusions. We developed a nomogram incorporating preoperative clinical data to predict the probability of SLNM development in high-risk cN0 PTC patients, which contributed to their optimized treatment options.
Key words: papillary thyroid cancer, nomogram, central lymph node metastasis, clinically node-negative (cN0), skip lymph node metastasis
Background
The metastasis of lymph node (LN) impacts surgical regimens and recurrence risk stratification in papillary thyroid cancer (PTC).1, 2 The dissemination of PTC cells through the lymphatic system occurs in a sequential pattern, involving the central compartment first, continuing to the ipsilateral lateral compartment, and finally metastasizing to the contralateral, lateral or mediastinal compartments.3 Skip lymph node metastasis (SLNM), on the other hand, is a rare form of lymph node metastasis (LNM). It metastasizes to the lateral lymph node (LLN) without involving the central lymph node (CLN).4 Some microscopic nodal positivity may be difficult to detect with ultrasonography prior to surgery due to atypical imaging characteristics. These patients are misdiagnosed as clinically node-negative (cN0) even though they received central lymph node dissection (CLND) due to the presence of significant LNM risk factors. As a result, their metastatic risk will be defined as low, thus affecting the treatment plans. Lymph node metastasis is probable in these individuals following surgery, resulting in an unfavorable prognosis such as additional surgery, radiotherapy, etc.5, 6, 7
Previous studies have found the independent correlates of SLNM, such as tumor in the upper pole of the thyroid gland and older age, and developed several prediction models for discerning patients with SLNM from those with typical LNM.8, 9, 10 However, the clinical utility of these models is limited because they are only applicable to assess the likelihood of SLNM in clinically node-positive (cN+) PTC, not cN0 PTC. In the abovementioned studies, surgical procedures and treatment strategies were identical for cN+ patients with or without SLNM. Although discerning SLNM from cN0 PTC may provide more individualized treatment strategies, including prophylactic lymph node dissection or closer nodal follow-up, we are currently unable to accurately determine the likelihood of SLNM in these high-risk cN0 PTC patients.
Objectives
The goal of this study was to establish a nomogram to preoperatively predict the likelihood of SLNM development in cN0 PTC patients who are at an increased risk of LNM, thereby optimizing their therapeutic options.
Materials and methods
The present study complied with the principles stated in the declaration of Helsinki. The institutional review board of the People’s Hospital of Yuhuan approved this study (approval No. YYLS2018(015)), and informed consent was obtained from all patients.
Study population
This study retrospectively analyzed medical records of 1836 newly diagnosed primary PTC patients undergoing thyroidectomy along with bilateral CLND, including cN+ patients and cN0 patients with an increased risk of metastasis and recurrence (e.g., larger tumor size, multifocal disease, extrathyroidal extension, family history of thyroid cancer, etc.). These medical records were obtained for patients admitted from January 2015 to May 2020. The tumor stage (tumor–node–metastasis (TNM)) in all patients was T1–4N0–1bM0 according to the 2015 American Joint Committee on Cancer (AJCC) guidelines.2 Patients with cN+ stage and central lymph node metastasis (CLNM), cN0 stage and CLNM, poorly differentiated papillary cancer, history of neck surgery, and history of radiotherapy were excluded.
All included patients had complete pathology reports and were regularly followed up for at least 2 years (until they were diagnosed with lateral lymph node metastases (LLNM) after surgery (endpoint event) or lost to follow-up). The follow-up deadline was June 30, 2022. Ultimately, 780 patients were included in this study (median follow-up 42 months, with 28 patients lost to follow-up (3.5%)). Based on the postoperative pathology reports and follow-up outcomes, cN+ patients without CLNM and cN0 patients with postoperative LLNM were assigned to the SLNM group (n = 82), and the CLNM-negative cN0 patients without postoperative LLNM into the non-SLNM group (n = 698) (Figure 1).
Data collection
The preoperative clinical data encompassing age, gender, body mass index (BMI), tumor (T) stage classified according to the tumor-node-metastasis (TNM) system, history of Hashimoto’s thyroiditis, and family history of thyroid cancer were obtained through the hospital information system. Tumor location, size, number of lesions, lesion distribution, hypoechoic mass, capsular invasion, extraglandular invasion, calcified foci, and Doppler blood flow were all examined and recorded with MyLab Class C ultrasonography (Esaote, Genoa, Italy).
Nomogram establishment
By investigating the differences in clinical indicators between the SLNM and non-SLNM groups, the variables with statistically significant differences between the 2 groups – that is, those with false discovery rate (FDR)-adjusted p-values <0.05 calculated using Benjamini–Hochberg correction – underwent univariate and multivariate Cox regression analyses to screen for the independent correlates of SLNM. A nomogram model was established based on these independent factors to predict the likelihood of SLNM in high-risk cN0 PTC patients before surgery.
Statistical analyses
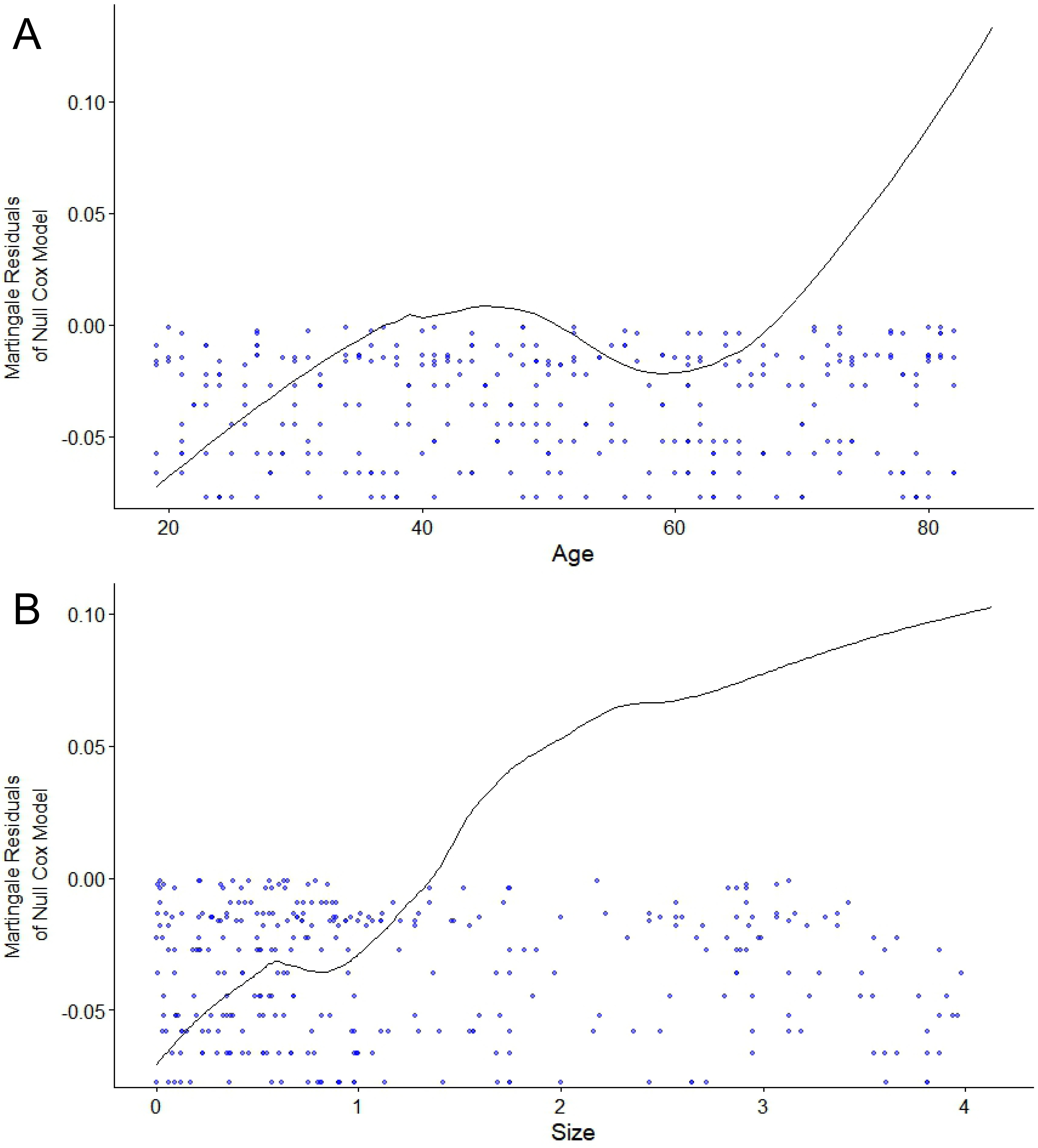
Independent-sample t-test or Mann–Whitney U test were employed to compare the continuous variables, depending on whether the distribution was normal or not (Shapiro–Wilk test). The homogeneity of variance was verified using Levene’s test prior to the t-tests. Categorical variables were compared using χ2 tests, and if the expected count in any table cell was less than 5, Fisher’s exact test was applied. For ranked data, Mann–Whitney U test was used. Multivariate Cox regression was used to obtain the hazard ratio (HR) and 95% confidence interval (95% CI) of each independent predictor utilized to develop the nomogram. For the Cox proportional hazards regression model, we assessed the validity of the proportional hazards assumption using Schoenfeld residuals and the linearity assumption using Martingale residuals for each continuous variable against survival time. Bootstrap resampling was used to adjust the overfitting deviation (1000 times). The concordance index (C-index) and Hosmer–Lemeshow (H–L) test were used to evaluate the discrimination and calibration of the model. The area under the receiver operating characteristic (ROC) curve (AUC) and the calibration curve were used to show the distribution of the discrimination and calibration. Decision curve analysis (DCA) was applied to assess the clinical applicability of the model. IBM SPSS v. 22.0 (IBM Corp., Armonk, USA), MedCalc v. 22.0.22 (MedCalc Software Ltd, Ostend, Belgium) and R package v. 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical analyses.
Results
Clinical characteristics in the SLNM and non-SLNM groups
Table 1 presents the clinical data comparisons between the SLNM and non-SLNM groups. Only BMI was compared using a t-test because it satisfied both normal distribution and variance homogeneity (p = 0.309 and 0.320, Shapiro–Wilk test; p = 0.533, Levene’s test). Regarding the comparison of categorical variables, χ2 tests were applied since no expected count in any table cell was less than 5 (Table 2).
Compared with the non-SLNM group, patients in the SLNM group were older and had a larger tumor size (both FDR-adjusted p-values <0.05). The proportions of tumor in the upper pole, single lesion, capsular invasion, and extraglandular invasion in the SLNM group were 46.34%, 71.95%, 67.07%, and 42.68%, respectively, and were all significantly higher than in the non-SLNM group (all FDR-adjusted p-values <0.05). There were no significant differences in gender, BMI, family history of thyroid cancer, history of Hashimoto’s thyroiditis, tumor T stage, lesion distribution, calcified foci, Doppler blood flow, and hypoechoic mass between the 2 groups (all FDR-adjusted p-values >0.05).
Univariate and multivariate analyses
for SLNM in PTC patients
Age, tumor location, number of lesions, tumor size, capsular invasion, and extraglandular invasion were the included variables for univariate Cox regression. They all satisfied the proportional hazards assumption (Figure 2). Among them, age and tumor size as continuous variables met the linearity assumption as well (Figure 3). Univariate analyses showed that they were all associated with the development of SLNM (all p < 0.05) and were therefore included in the multivariate analysis. Multivariate Cox regression analysis revealed that older age, tumor in the upper pole, larger tumor size, and capsular invasion were the independent correlates of SLNM (all p < 0.05; Table 3).
Nomogram development
A nomogram was built based on the 4 independent predictors (age, tumor location, tumor size, and capsular invasion; Figure 4). The estimated risk of SLNM was calculated by summing the points of each predictor, with the weight equal to the HR values. The risk range of SLNM was 20–60% in this nomogram.
Nomogram verification
To evaluate the accuracy of the nomogram, a high AUC was obtained (0.824 > 0.75) (95% CI: 0.891–0.964) after 1000 bootstrapping to adjust overfitting deviation in the ROC analysis, indicating a good discrimination (Figure 5).
Evaluation of the calibration
and clinical net benefit
The agreement between the probability of SLNM development predicted using the nomogram and the actual incidence was evaluated with the calibration curve and the H–L test. It showed good agreement with the actual incidence when the predicted probability was <60% (χ2 = 5.263, p = 0.729, H–L test; Figure 6A). The DCA curve showed that the probability predicted using the nomogram obtained a net benefit when the probability was <60% (Figure 6B). It indicated that the prediction model could reliably identify high-risk cN0 PTC patients with SLNM.
Discussion
The SLNM in CLNM-negative cN0 patients affects the clinical stage and recurrence risk stratification. In this study, we revealed the independent correlates of the development of SLNM (age, tumor location, tumor size, and capsular invasion) by analyzing the clinical characteristics of cN+ patients without CLNM (typical SLNM) and cN0 patients with postoperative LLNM (occult SLNM). Based on these predictors, a nomogram was established to preoperatively predict the likelihood of SLNM development in cN0 PTC patients who are at an increased risk of LNM, allowing for more individualized therapy.
The presence of SLNM is not detected with CLND. Sometimes, it is invisible for ultrasonography in an early stage due to atypical imaging features, leading to these patients being mistakenly classified as low-risk.10 Patients with occult SLNM are more likely to experience postoperative disease progression, which may lead to an unfavorable prognosis such as additional surgery.11 The incidence of postoperative metastasis in cN0 PTC may be successfully lowered if these patients are identified prior to surgery, thus improving the prognosis.11, 12 The majority of studies on SLNM analyzed the characteristics of cN+ patients compared to subjects without SLNM, while cN0 PTC patients were not enrolled.8, 10, 13 Despite the fact that Yang et al. included cN0 PTC patients in their study, they determined that cN0 PTC patients were all non-SLNM patients and did not conduct postoperative follow-up, ignoring the occult SLNM in cN0 PTC.14
Some of the possible reasons for postoperative LLNM in cN0 PTC patients are: 1) occult SLNM is present prior to surgery, and residual tumor cells in the LLN induce postoperative LLNM15; or 2) after CLND, tumor cells in the residual thyroid gland metastasize to the LLNs via the lymphatic pathway along the upper pole of thyroid vessels vasculatures because the lymphatic pathway to CLNs is blocked.16 Therefore, we believe that postoperative LLNM in cN0 PTC patients who received CLND is mostly SLNM. In this study, postoperative follow-up was performed to identify occult SLNM patients in order to investigate the risk factors of SLNM and establish a prediction model to estimate the risk of SLNM.
Multivariate Cox regression analysis in this study found that older age, larger tumor size, upper pole location, and capsular invasion were independent factors of the SLNM development. Older age is a risk factor for both PTC17, 18, 19 and LLNM.20, 21 Wang et al. concluded that age ≥55 years was an independent risk factor for SLNM, which is consistent with the findings of this study.13 There is still controversy regarding the size and invasiveness of tumors in SLNM patients. Wang et al. and Lim et al. found that SLNM was more likely to develop in weakly invasive PTC, and that tumor size <1 cm was an independent risk factor.13, 22 However, Yang et al. showed that tumor size >1 cm and capsular invasion were independent factors for SLNM.14 Our results support the idea of Yang et al.14 that large tumor size and capsular invasion are independent factors for the development of SLNM, and these factors are similar to the risk factors for LLNM.23, 24, 25 One possible explanation is that a tumor with a larger diameter and capsular invasion may be closer to the lymphatic pathway along the upper pole of thyroid vessels, allowing tumor cells to directly metastasize to the LLN. In this study, tumors in the upper pole of the gland were found in 46.34% of SLNM patients, which was higher than in the non-SLNM group. It is in line with the studies by Yang et al.14 and Hou et al.26 The possible explanation is that the lymph from the upper pole of the thyroid mainly enters the venous system through the lateral cervical lymph nodes along the lymphatic vessels accompanying the superior thyroid artery.16, 27 Therefore, the tumor cells located in the upper pole of the thyroid are more susceptible to spread to the LLNs through the ascending lymphatic vessels, leading to SLNM.
To our knowledge, this is the first study to establish a nomogram for quantifying the likelihood of SLNM development in high-risk cN0 PTC patients before surgery. The model was confirmed to have good discrimination after resampling. When the predicted probability was <60%, the model agreed well with the actual incidence and could yield a net benefit. Clinicians can assess the likelihood of SLNM in each high-risk cN0 PTC patient before surgery by utilizing this nomogram. For example, when a 50-year-old patient with a 3-centimeter invasive tumor in the upper pole of the thyroid has a calculated score of approx. 220 points, it means that the likelihood that this patient will develop SLNM is more than 50%, and prophylactic lymph node dissection or closer nodal follow-up may be recommended.
Limitations
There are some limitations to this report. First, because it was a retrospective study with a limited sample, we did not have the opportunity to assess the angioinvasion of lymphatic vessels, or perform an external validation to further evaluate the performance of the model. Second, limited follow-up time could result in missing patients with postoperative metastases due to occult SLNM. Finally, we were unable to analyze the occult SLNM in low-risk cN0 PTC patients because pathological results of their CLNs were not available. We plan to conduct a multi-center prospective study with a longer follow-up period in order to strengthen the prediction model.
Conclusions
We developed a nomogram which incorporated age, tumor location, tumor size, and capsular invasion, for preoperatively estimating the likelihood of SLNM development in cN0 PTC patients at an increased risk of LNM. If further validated, it will allow for personalized therapies to minimize the incidence of postoperative metastases in patients with cN0 PTC.