Abstract
This editorial explores the clinical potential of complete blood count-derived inflammation indices (CBC-DIIs) as sensitive and cost-effective measures of systemic inflammation and immune response.
Key words: immune response, systemic inflammation, complete blood count, CBC-derived indices, CBC parameters
Introduction
Systemic inflammation is central to developing and progressing diverse diseases, including malignancies, cardiovascular diseases (CVD) and chronic inflammatory conditions. Traditionally, biochemical markers have been used to assess inflammation; however they often fail to capture the dynamic interplay of immune responses while incurring higher costs and complexity.
Several ratios and composite multiparametric indices of biochemical and hematological markers are used to assess inflammation, disease severity and prognosis in various conditions, like albumin-to-globulin ratio, C-reactive protein (CRP) to albumin ratio, comprehensive inflammation index, CRP to lactate dehydrogenase ratio, fibrinogen-to-albumin ratio (FAR), Glasgow prognostic score (GPS), monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), CD4/CD8 ratio, CD8/CD56 ratio, interleukin (IL)-1β/IL-10 ratio, IL-10/tumor necrosis factor alpha (TNF-α) ratio, etc.
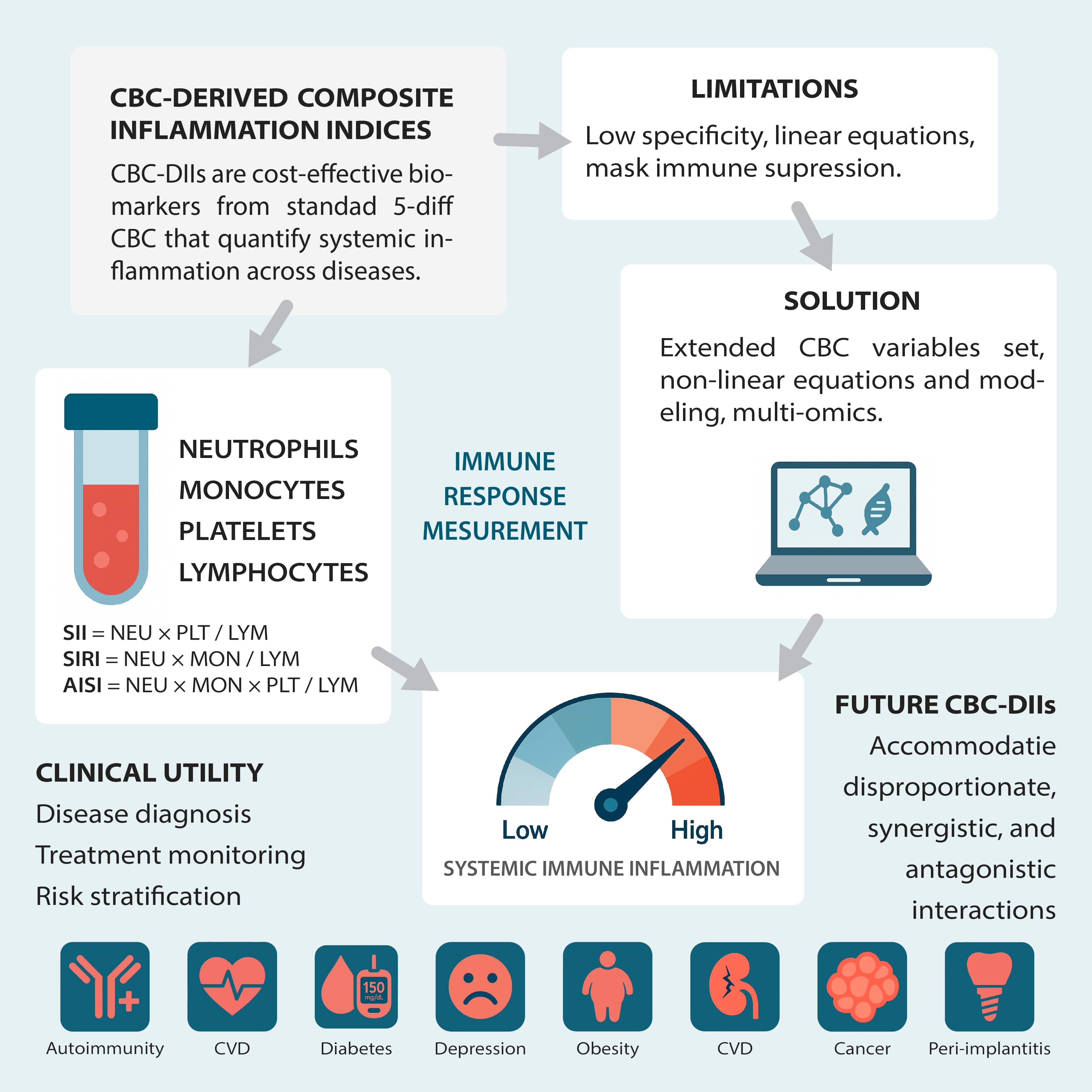
The complete blood count (CBC), a routine and cost-effective test, offers an attractive alternative. Composite indices derived from standard CBC parameters enable a sensitive and rapid assessment of systemic immune activation. We focus on 3 such indices – the Systemic Immune-Inflammation Index (SII), Systemic Inflammation Response Index (SIRI) and Aggregate Index of Systemic Inflammation (AISI). These indices integrate measurements of neutrophils (NEU), platelets (PLT), monocytes (MON), and lymphocytes (LYM) to reflect the equilibrium between pro-inflammatory and anti-inflammatory processes. The discussion encompasses the biological mechanisms behind these indices, their clinical relevance as diagnostic and prognostic tools, and the limitations of their current linear models, setting the stage for future nonlinear and integrative diagnostic frameworks.
This editorial is intended for clinicians and biomedical researchers involved in diagnosing, prognosis and managing inflammation-related diseases. For clinicians, it highlights the practical and actionable potential of CBC-derived inflammation indices (CBC-DIIs), which are readily available, cost-effective and underutilized in routine practice. By highlighting their role in common diagnostic pathways and monitoring strategies, we aim to promote more confident clinical adoption. For researchers, we call attention to the field’s methodological stagnation. Despite the increasing number of validation studies, a pressing need remains for innovation through systems biology, nonlinear modeling and integration with genomic and imaging data. These advances could transform CBC-DIIs into mechanistically insightful and computationally robust tools for personalized medicine.
The following sections discuss the biological mechanisms behind these indices, their clinical relevance and the limitations of their current linear models, setting the stage for an integrative diagnostic framework.
The mechanisms behind CBC-derived indices in inflammation assessment
The foundation of CBC-DIIs lies in the dynamic interplay between circulating cytokines,1 hematopoiesis,2 blood cell counts and their activation.3, 4 For clinicians, these shifts manifest as measurable changes in routine CBC parameters (Table 1, Table 2), such as neutrophilia or thrombocytosis, providing an indirect but meaningful reflection of underlying immune activity.
Increased levels of pro-inflammatory cytokines can lead to cell activation, proliferation and increased counts (e.g., neutrophilia, thrombocytosis), while anti-inflammatory cytokines can reduce activation and maintain immune homeostasis. Each cytokine has distinct and overlapping effects, sometimes promoting or inhibiting the proliferation or activation of blood cells in response to specific immune challenges (Table 3, Table 4). Understanding these mechanisms enhances clinicians’ ability to interpret elevated indices in specific clinical contexts, such as distinguishing between infection and autoimmunity or assessing treatment response.
From a research standpoint, this mechanistic view provides fertile ground for biomarker development. Each cytokine exerts distinct effects on hematopoietic lineages, often in overlapping or opposing ways. Aggregating cell counts into composite indices captures the net effect of these signals but lacks resolution in the specific pathways involved. Advanced studies that integrate CBC-DIIs with cytokine profiling or transcriptomic analyses could offer deeper insights into the biological mechanisms underlying immune imbalance and disease progression.
Composite indices based on CBC: SII, SIRI and AISI
Building on these mechanistic insights, 3 primary composite indices have been developed, each offering distinct value in assessing systemic inflammation. For clinicians, these shifts are reflected in measurable changes in routine CBC parameters (Table 1, Table 2), such as neutrophilia or thrombocytosis, offering an indirect yet meaningful indication of underlying immune activity.
Systemic immune-inflammation index was developed by Hu et al.5 in 2014. The SII incorporates the absolute counts of NEU, PLT and LYM using the following formula, with counts expressed as ×109 cells/L:
SII = (NEU × PLT)/LYM
Systemic immune-inflammation index captures the interplay between pro-inflammatory cells (NEU and PLT) and anti-inflammatory LYM. Elevated SII values typically indicate a predominance of pro-inflammatory activity, commonly observed in conditions such as cancer and CVD, where a high SII is associated with adverse outcomes.6, 7, 8, 9
The SII was introduced by Qi et al.10 The SIRI comprises NEU, MON and LYM, reflecting the involvement of innate and adaptive responses in inflammation. The formula, with counts expressed in ×109 cells/L, is:
SIRI = (NEU × MON)/LYM
The SIRI incorporates parameters that reflect the inflammatory response (such as NEU, MON and LYM count). The SIRI is particularly useful in diseases with a significant inflammatory component, including cancer, infections and trauma. Clinicians may find SIRI helpful in infectious and trauma settings, where rapid immune shifts are pronounced.
The AISI,10 which combines the components of both SII and SIRI, integrates NEU, MON, PLT, and LYM into a single index with counts expressed as ×109 cells/L:
AISI = (NEU × MON × PLT)/LYM
The AISI combines these ratios and other inflammatory markers to give a more comprehensive measure of systemic inflammation. It is a robust tool for diagnosing and monitoring diseases with an inflammatory component. Its integration of multiple parameters may enhance specificity in complex cases.
In 2020, Fuca et al.11 published the same equation, apparently specifically named pan-immune-inflammation value, to reflect its broad applicability across various disease states, particularly in oncology. Adhering to publication priority reasons, we use the AISI abbreviation to denote the index.
Despite their sensitivity, these indices rely on static, linear relationships that may oversimplify the inherently dynamic, nonlinear nature of immune interactions, leading to limitations in specificity.
From a research perspective, these indices provide a framework for capturing systemic immune status; however, their reliance on linear relationships constrains their application. This simplification overlooks threshold effects, feedback loops and synergistic interactions that are common in biological systems. As such, there is a growing need to transition toward nonlinear, model-driven approaches that more accurately reflect immune complexity.
The reciprocal diseases–systemic inflammation axis
Systemic inflammation and disease are interconnected in a reciprocal relationship. Self-amplifying cycle, where chronic pathological states fuel immune activation, and inflammation further exacerbates disease progression. For clinicians, this dynamic underscores the importance of monitoring inflammation as a consequence of disease and as a therapeutic target. Chronic conditions such as cancer, CVD and autoimmune disorders drive systemic inflammation, which in turn accelerates disease progression. Inflammation is not just a consequence of disease but a key mechanism that accelerates disease progression. Once systemic inflammation is established, it contributes to disease progression by disrupting signaling and metabolic pathways, leading to organ dysfunction and complications. Inflammation actively amplifies disease processes rather than merely reflecting them.
Clinical factors – such as comorbidities, infections, medications, stress, and environmental factors, also influence systemic inflammation. Although various clinical and external factors can influence inflammatory biomarkers – complicating their interpretation – these influences also offer important context for understanding the role of inflammation in disease. Incorporating such variables makes CBC-DIIs more applicable to real-world clinical settings, where ongoing interactions between disease processes and patient-specific factors are the norm.
The bidirectional relationship between disease and systemic inflammation underscores a critical gap in current research – the need for precise quantification of inflammatory activity and its feedback effects on disease progression. To accurately predict outcomes, it is imperative to quantify systemic inflammation in the context of both the underlying pathology and its external modulators. Composite indices – comprising diverse markers, such as cytokine panels, CBC parameter ratios, and metabolomic signatures – should be integrated into dynamic, nonlinear modeling frameworks and rigorously validated in longitudinal cohorts to provide a comprehensive view that augments prognostication and informs therapeutic decisions. Current models often overlook the cumulative and context-specific effects of inflammation over time. Consequently, future research should employ time-series data, systems modeling, and multimodal integrative diagnostics to elucidate these intricate interactions and provide a more comprehensive understanding of how immune dynamics influence clinical outcomes.
Clinical applications of CBC-derived indices
The CBC-DIIs such as the SII, SIRI and AISI have demonstrated utility across a diverse range of clinical conditions beyond oncology and CVD.6, 9, 12, 13, 14 Elevated SII, SIRI and AISI values are correlated with disease risk, prevalence and severity in various conditions.13, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 In oncology, elevated SII values are associated with a tumor microenvironment that facilitates immune evasion and tumor progression, and they are strongly correlated with poor prognosis.6, 7, 9, 26 Elevated SII levels have been associated with increased severity of depression, suggesting its potential as an auxiliary diagnostic indicator in depressive disorders.21, 27 Similarly, in patients undergoing maintenance hemodialysis, high SII has been identified as an independent risk factor for depression, highlighting its relevance in neuropsychiatric assessments within chronic illness populations.28
In dental health, higher SII values have been observed in patients with generalized stage III grade C periodontitis than in healthy individuals.29 This association underscores the SII and SIRI utility in identifying systemic inflammation linked to periodontal disease.22, 30
The Systemic Immune-Inflammation Index, SIRI and AISI have been found to correlate with disease activity in autoimmune disorders, such as psoriatic arthritis, ankylosing spondylitis, systemic lupus erythematosus, Sjögren’s syndrome, and autoimmune encephalitis, indicating their potential as biomarkers for monitoring disease severity.18, 31, 32, 33, 34, 35
Studies have demonstrated a positive association between elevated SII levels and the incidence of chronic kidney disease (CKD) in adults, particularly in men. This suggests that SII could serve as a valuable marker for early identification and risk stratification in CKD.36, 37
The SII has shown promise in differentiating between active and inactive disease states in infectious diseases, as evidenced by its effectiveness in distinguishing active pulmonary tuberculosis from non-tuberculous lung diseases.38 In acute settings, such as sepsis,24 serial measurements of these indices can provide real-time insights into the evolving inflammatory state, thereby guiding timely therapeutic interventions.
Elevated SII levels have been associated with an increased risk of type 2 diabetes mellitus (T2DM) and insulin resistance. Studies have found that higher SII levels are independently linked to elevated fasting plasma glucose, fasting insulin and HOMA-IR values, indicating a higher risk of developing T2DM and insulin resistance.16, 39 Furthermore, in patients with diabetic retinopathy, high SII values predicted both microvascular and macrovascular complications, as well as increased mortality risk within the first year, highlighting its potential as a prognostic marker in diabetic complications.40
In the context of acute trauma, particularly traumatic brain injury (TBI), SII has been identified as a valuable prognostic biomarker. A retrospective study involving 1,266 patients with severe TBI demonstrated that elevated SII levels at admission were independently associated with poorer outcomes, including higher mortality rates and unfavorable Glasgow Outcome Scores at 6 months post-injury.41 Similarly, in pediatric TBI cases, significant differences in SII values were observed across mild, moderate and severe injury groups, suggesting its utility in assessing injury severity and guiding clinical management.42
These examples illustrate the broad applicability of CBC-DIIs in various clinical scenarios, providing clinicians with accessible tools for prognostication and aiding in the development of personalized patient management strategies.
While CBC-DIIs are invaluable in clinical practice, their interpretation should always be contextual, as elevated values may also occur in benign inflammatory responses. Consequently, they must be used in conjunction with other diagnostic tests and clinical assessments to ensure accuracy.
The future of advanced CBC-DIIs: Nonlinear interactions and advanced framework
Integrating routinely acquired imaging data – such as radiomic texture and histopathological classification (including architectural patterns, cytological variants, and grading) – with broad genomic signatures like transcriptomic profiles provides the complex, context-specific inputs that nonlinear models need to reveal threshold phenomena, feedback loops, and synergistic interactions driving immune and inflammatory responses – capabilities that linear CBC-based indices inherently lack.43 Biological systems often exhibit threshold effects, feedback loops and context-dependent responses – phenomena that linear models cannot adequately represent. Small perturbations in one immune parameter can result in disproportionate downstream effects, a nuance that linear models are unequipped to handle.44 This is frequently highlighted as a relationship that is roughly linear or a nonlinear relationship between CBC-DIIs, mortality risk, disease risk or severity, organ health, or other clinical indices.20, 22, 36, 45, 46, 47, 48, 49, 50, 51, 52, 53 Nonlinear modeling techniques offer a more flexible and biologically congruent framework, accommodating disproportionate, synergistic or antagonistic interactions among immune variables.
For instance, discrete shifts in blood cell counts and their parameters have varying impacts on different individuals. These nonlinearities can be captured using mathematical expressions that model each immune component as a distinct, interacting “factor,” rather than assuming uniform additive or subtractive contributions. Factors can include immune activation states, regulatory capacity or systemic burden, each constructed from multi-variable sub-formulas that allow nonlinear responses to subtle changes in input data.
To operationalize such models, computational methods such as decision trees,54 random forests55 or gradient boosting56 can be employed. These algorithms are particularly well-suited for identifying complex decision boundaries, such as distinguishing between immune activation and immune suppression in ambiguous or overlapping CBC profiles. More advanced approaches, such as support vector machines (SVMs)57 or artificial neural networks,58 can model high-dimensional interactions and uncover patterns that would otherwise remain obscured in linear frameworks. These techniques address multicollinearity and feature interaction and can be trained on real-world patient data to adapt their predictions to different clinical scenarios dynamically.
Moreover, incorporating temporal dimensions through recurrent neural networks (RNNs)57 or long short-term memory networks (LSTM)58 would enable the indices to account for how immune parameters evolve, thereby enhancing prognostic accuracy in chronic conditions or for monitoring treatment responses. Such time-aware models could flag abnormal trajectories even when snapshot values appear clinically acceptable.
Ultimately, nonlinear and machine learning-based frameworks offer the precision, adaptability and sensitivity needed to transform CBC-DIIs from static screening tools into dynamic, personalized biomarkers. They are especially valuable in contexts with immune suppression, comorbidity burden or fluctuating disease activity, where linear assumptions often fail. By embracing these advanced modeling strategies, future CBC-DIIs should more accurately reflect immune complexity, thereby supporting early diagnosis, risk stratification and tailored intervention.
Integration of CBC-derived indices with multimodal diagnostics
The integration of CBC-DIIs with other diagnostic modalities presents a promising area for enhancing personalized assessments of immune function. In accordance with the nonlinear, multimodal modeling framework outlined above, the following sections detail five domains – radiographic imaging and radiomics; genomic and transcriptomic profiling; biochemical laboratory markers; proteomic and metabolomic analyses; and bioelectrical impedance assessment – in which CBC-DIIs are integrated with established diagnostic modalities to improve the sensitivity and specificity of immunological personalized evaluations. Below, we present some illustrative examples of such integrations.
Radiographic imaging and radiomics: magnetic resonance imaging (MRI) and computed tomography (CT) scans offer detailed anatomical and functional insights, which, when combined with CBC-DIIs, enhance the evaluation of inflammatory and neoplastic conditions. For example, in multiple sclerosis, MRI improves the assessment of disease activity and progression.59 Radiomics, which extracts quantitative features from medical images, further enhances this integration by correlating imaging phenotypes with hematological indices, thereby refining prognostic models in oncology and other fields.
Genomic and transcriptomic data: The incorporation of genomic and transcriptomic data with CBC-DIIs facilitates a deeper understanding of the molecular underpinnings of immune responses. Genetic variations influencing immune cell function can modulate blood cell phenotypes, and their integration can aid in identifying individuals at risk for autoimmune diseases or adverse drug reactions. Transcriptomic profiling, in conjunction with CBC-DIIs, can also assist in monitoring disease activity and therapeutic responses in conditions such as rheumatoid arthritis and systemic lupus erythematosus.
Biochemical laboratory results: Combining CBC-DIIs with biochemical markers, such as adipokines and bone metabolism markers, would enhance the evaluation of systemic inflammation, obesity and bone health. Such a multimodal approach improves the sensitivity and specificity of diagnostic algorithms by providing a more nuanced picture of the patient’s inflammatory status.
Omics data integration: Integrating proteomics, metabolomics and other omics data with CBC-DIIs provides a holistic view of the immune system’s status, potentially identifying novel biomarkers and pathways involved in disease processes. This comprehensive profiling can lead to personalized medicine approaches, e.g., metabolomic profiles combined with CBC-DIIs could improve the prediction of metabolic syndrome risk in high-risk populations.
Bioelectrical impedance analysis (BIA) provides non-invasive measurements of body composition, including fat mass, lean body mass and total body water, which are crucial for understanding metabolic and inflammatory status. Combined with CBC-DIIs, clinicians can gain a more comprehensive view of patients, especially those with systemic inflammation and conditions like obesity and sarcopenia.
While the integration of CBC-DIIs with other diagnostic modalities holds significant promise, challenges remain. Data standardization, interoperability and the development of robust analytical frameworks are essential for effective integration. Adopting standardized data formats and leveraging machine learning algorithms can facilitate the synthesis of diverse data types, leading to more accurate and individualized assessments of immune function. Without adherence to interoperability standards such as Fast Healthcare Interoperability Resources (HL7/FHIR; https://hl7.org/fhir/exchange-module.html), efforts to unify data across health systems may falter. Moreover, as models grow more complex, ensuring clinical interpretability becomes essential to avoid creating opaque decision tools that hinder, rather than support, frontline care.
Future directions
Future research should extend beyond CBC-DIIs dominating applications in oncology to fully harness the clinical and diagnostic potential of advanced multiparametric CBC-DIIs. Several underexplored domains offer opportunities for investigation.
In metabolic disorders such as obesity, where chronic low-grade inflammation is a hallmark,52, 60, 61 The CBC-DIIs could aid in diagnosing and quantifying the systemic inflammatory burden linked to comorbidities,48 as well as monitoring the efficacy of interventions. In depression, systemic immune activation is increasingly recognized as a contributing factor62; dedicated CBC-DIIs may provide objective markers to support diagnosis, predict treatment responsiveness and monitor residual inflammatory burden. In implant medicine, including dental implants63 and orthopedic joint replacements,64 specialized indices could serve as early indicators of immunosuppressive or inflammatory foreign body response, infection risk or long-term inflammatory complications. This would be especially valuable in enhancing pre- and post-operative surveillance. Allergic conditions may also benefit from adapted CBC-DIIs formulations that reflect acute and chronic immune activation.64, 65 Additionally, systemic oxidative stress often involves systemic inflammation and shifts in CBC counts and their parameters,66, 67 making a dedicated CBC-DII a potential tool for detecting and measuring the impact of oxidative stress on the immune system. In contexts of chronic immunosuppression, such as organ transplantation or chronic inflammatory diseases, dedicated CBC-DIIs could help identify atypical immune profiles. Likewise, nuanced index behavior may reveal suppressed or dysregulated immune response patterns indicative of emerging complications in primary or acquired immunodeficiencies.
A common limitation of currently available CBC-DSIIs is their lack of specificity, despite their sensitivity. These indices rely on static, linear relationships that may oversimplify the inherently dynamic and nonlinear nature of immune interactions, resulting in limitations in specificity. However, for clinical and research applications, new advanced CD-SIIs with enhanced condition or disease specificity may offer a superior advantage. For instance, in depression, the objective would be to identify specific blood cell ratio patterns associated with depression severity. In contrast, in obesity, the focus would be on identifying specific patterns of adipose tissue inflammation. Due to shared biological mechanisms of depression and obesity,68 research suggests that obesity is a causal risk factor for elevated risk of depression and increases the risk of depression,69, 70 and observational studies have provided some evidence for a bidirectional association, indicating that psychological distress may also contribute to an increase in body mass index (BMI).71 As a result, the development of distinct CD-SIIs specific to adipose tissue inflammation and depression would provide a significantly deeper understanding of the obesity–depression correlation. This would enable the creation of tools to quantify correlated systemic inflammation levels and monitor treatment responses.
Conclusions
The CBC-DIIs offer a robust and cost-effective method for assessing systemic inflammation by utilizing routine CBC tests. These indices provide sensitive measures of immune activation, aiding in the diagnosis, prognosis and monitoring of diseases ranging from cancer and CVD to autoimmune conditions. Despite their promise, the current linear models underpinning these indices do not fully capture the complexity of immune–inflammatory interactions, particularly in patients with chronic conditions or those who are immunocompromised.
Advancing these tools will require a shift toward nonlinear frameworks that reflect the dynamic, non-proportional nature of biological systems. Integrating these models with multi-modal data, such as imaging, genomics and longitudinal patient records, can dramatically improve diagnostic precision and personalization. However, such integration is not without challenges. Data from different sources vary in format, scale and clinical context, making harmonization and interpretation difficult without adherence to interoperability standards
For clinicians, future indices must remain accessible, interpretable and grounded in routine practice. For researchers, the imperative is to drive innovation in data modeling, integration and validation, ensuring that these tools are both biologically insightful and practically deployable. By embracing multi-disciplinary approaches and addressing these technical and clinical challenges, CBC-DIIs may evolve into truly personalized biomarkers of precision diagnostics in systemic inflammation.