Abstract
Cancer progression and therapeutic resistance are propelled by the remarkable plasticity of signaling networks, which dynamically rewire under selective pressures to maintain proliferation, enable immune evasion and promote metastasis. Despite advances in precision oncology, the dynamic crosstalk between tumor cells, non-coding genomes and the microenvironment continues to undermine treatment efficacy. This call for submissions, Revolutionizing Cancer Treatment: Navigating the Intricate Landscape of Cellular Signaling Networks, seeks cutting-edge research that dissects these adaptive mechanisms through innovative technologies – from single-cell multi-omics and spatial transcriptomics to AI-powered network modeling. We welcome studies leveraging physiomimetic models (e.g., organoids, 3D-bioprinted ecosystems) to decode tumor heterogeneity, as well as translational work targeting emergent vulnerabilities at the intersection of epigenetics, metabolic reprogramming and stromal interactions. By integrating systems biology with computational and experimental approaches, this collection aims to catalyze the design of adaptive therapies that outmaneuver cancer’s evolutionary resilience.
Key words: cancer treatment, cellular signaling networks, biomarkers
Introduction
Cancer, a disease of profound complexity, arises from the dysregulation of cellular signaling networks that orchestrate cell fate, immune evasion and metastatic dissemination.1 While chemotherapy, immunotherapy and targeted therapies have transformed oncology, the adaptability of tumors – fueled by redundant pathways, microenvironmental interactions and evolutionary pressures – remains a formidable barrier. This call for submissions, Revolutionizing Cancer Treatment: Navigating the Intricate Landscape of Cellular Signaling Networks, invites the scientific community to confront the complex challenges of cancer research through a systems-level lens – bridging molecular discovery with translational innovation.
The double-edged sword of signaling complexity: From pathways to networks
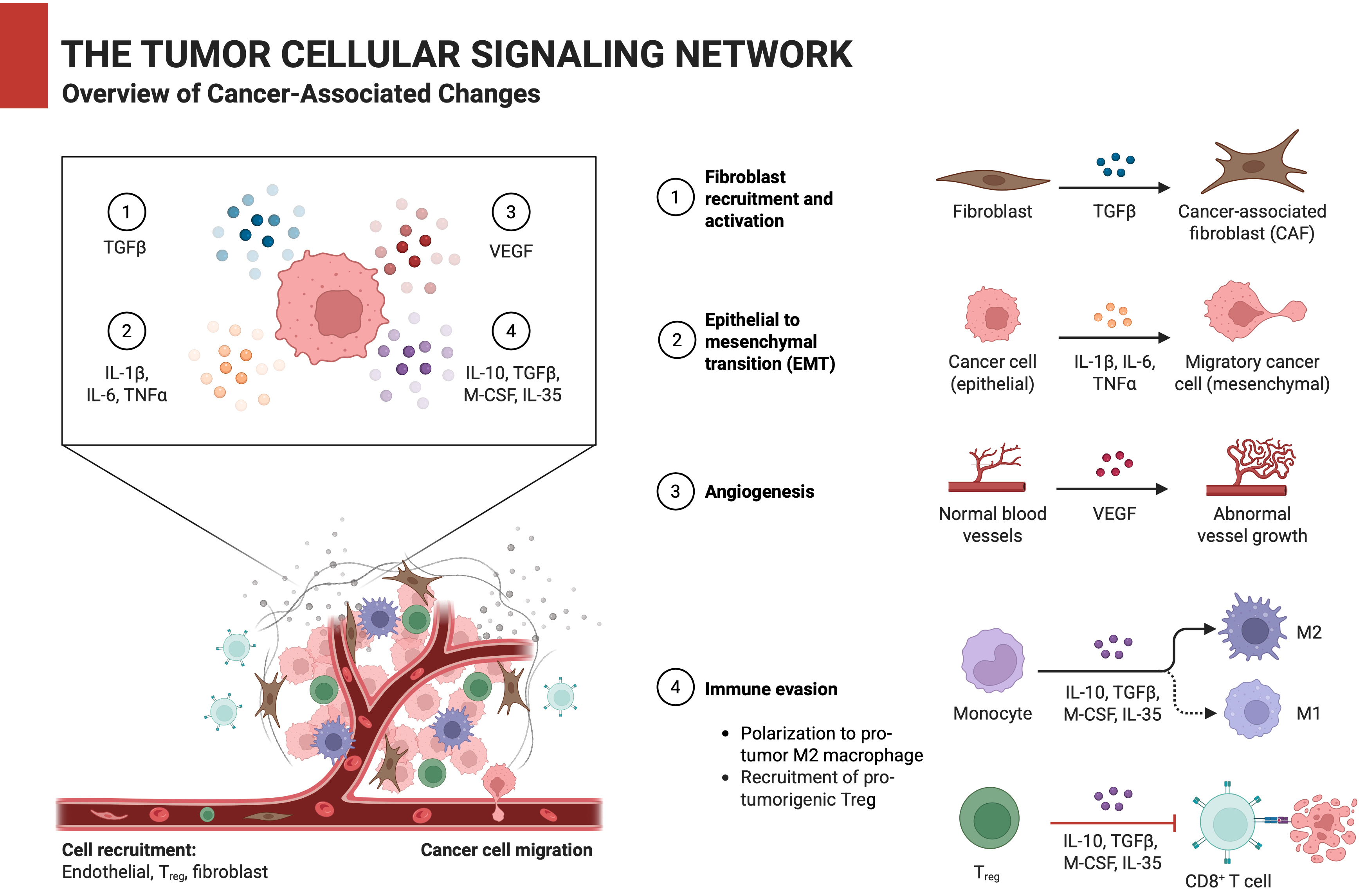
Carcinogenesis is not merely the result of driver mutations, but rather a systemic collapse of signaling homeostasis. Canonical pathways such as PI3K/AKT/mTOR,2 RAS/MAPK,3 and Wnt/β-catenin4 are frequently co-opted by tumors; however, their extensive crosstalk and functional redundancy often create escape routes that undermine therapeutic efficacy. For example, BRAF (B-Raf proto-oncogene, serine/threonine kinase) inhibitors in melanoma initially shrink tumors, only to see resistance emerge via NRAS (neuroblastoma RAS viral oncogene homolog) mutations or EGFR (epidermal growth factor receptor)-driven rewiring.5 Similarly, EGFR-targeted therapies in lung cancer fail when tumors activate AXL (AXL receptor tyrosine kinase) or MET (MET proto-oncogene, receptor tyrosine kinase) signaling.6, 7 Even immunotherapy, which reinvigorates cytotoxic T cells by blocking PD-1 (programmed cell death protein 1)/PD-L1 (programmed death-ligand 1), is thwarted by compensatory immunosuppressive networks involving TGF-β (transforming growth factor beta), adenosine or regulatory T cells within the tumor microenvironment (TME).8 The TME itself acts as a dynamic signaling hub. Cancer-associated fibroblasts (CAFs) secrete growth factors like FGF (fibroblast growth factor) and VEGF (vascular endothelial growth factor),9 while tumor-associated macrophages (TAMs) release interleukin 10 (IL-10) and CCL22,10 creating a pro-metastatic niche. Recent studies reveal that extracellular vesicles (EVs) shuttle oncogenic miRNAs (e.g., miR-122) between tumor and stromal cells, further entrenching resistance.11 These interactions underscore the need to map signaling networks as adaptive circuits rather than static pathways.
Bridging the gap: From multi-omic insights to AI, spatial biology and beyond
Advances in multi-omics – proteogenomics,12 spatial transcriptomics13 and metabolomics14 – have unmasked tumor heterogeneity and plasticity.15 Single-cell RNA sequencing has identified rare subpopulations with enhanced tumor metastasis ability.16 Furthermore, computational models now simulate network responses to perturbations, predicting resistance mechanisms and optimal drug sequences. Artificial intelligence (AI) and machine learning (ML) are accelerating discoveries. For instance, the deep learning model identified 8 core protein assemblies integrating multi-gene alterations to predict palbociclib response in breast cancer, outperforming single-gene biomarkers.17 Spatial transcriptomics, spatial proteomics and computational approaches have been combined to reveal that gliomas organize into spatially structured cellular states, with local microenvironments dominated by single states and specific state pairs consistently co-localizing across tumors. Hypoxia emerges as a key driver of long-range tissue architecture, shaping a layered global organization absent in non-hypoxic tumors like low-grade IDH-mutant gliomas.18 Patient-derived organoids19 and three-domesnional (3D) bioprinted TME20 models are also revolutionizing preclinical testing. These platforms recapitulate stromal interactions and drug penetration barriers, enabling high-throughput screening of network-targeted therapies.
Call for contributions
We invite contributions that explore the following emerging and advanced areas of cancer biology and therapy:
– Novel regulators of oncogenic hubs: Non-coding RNAs, phase-separated condensates and post-translational modifiers (e.g., ubiquitin ligases).
– TME-mediated network modulation: Role of exosomes, circadian rhythm disruptions and neural signaling in metastasis.
– Resistance mechanisms: Epigenetic plasticity, adaptive kinome reprogramming and persister cell states.
– AI/ML-driven discovery: Network-based drug repurposing and digital twins for personalized therapy.
– Biomarkers: Circulating tumor DNA (ctDNA) for real-time network monitoring, and metabolic imaging signatures.
Conclusions
The future of cancer therapy lies in decoding the rules that govern signaling networks – not just the molecules. By integrating systems biology, AI, and spatially resolved technologies, we can design therapies that anticipate and disrupt cancer’s adaptive strategies. This call for submissions seeks to catalyze interdisciplinary collaboration, uniting basic researchers, computational biologists and clinicians to transform complexity into curability.