Abstract
Background. Heart failure (HF) is a chronic condition affecting tens of millions of people worldwide. Despite advances in treatment, its impact on mental health, cognitive function and self-care behaviors remains underexplored, particularly across ejection fraction phenotypes, underscoring the need for comprehensive investigations into these interconnected domains.
Objectives. This prospective cohort study investigated changes in affective symptoms, cognitive functioning and self-care behaviors in patients with HF stratified with ejection fraction (EF) phenotypes over 6 months.
Materials and methods. The study included 162 patients aged over 60 years with a diagnosis of HF. Participants were examined at enrollment and after 6 months. The Mini-Mental State Examination (MMSE), the Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire-9 (PHQ-9) and the European Heart Failure Self-care Behaviour Scale (EHFScB-9) were used to assess cognitive function, affective symptoms and self-care behaviors.
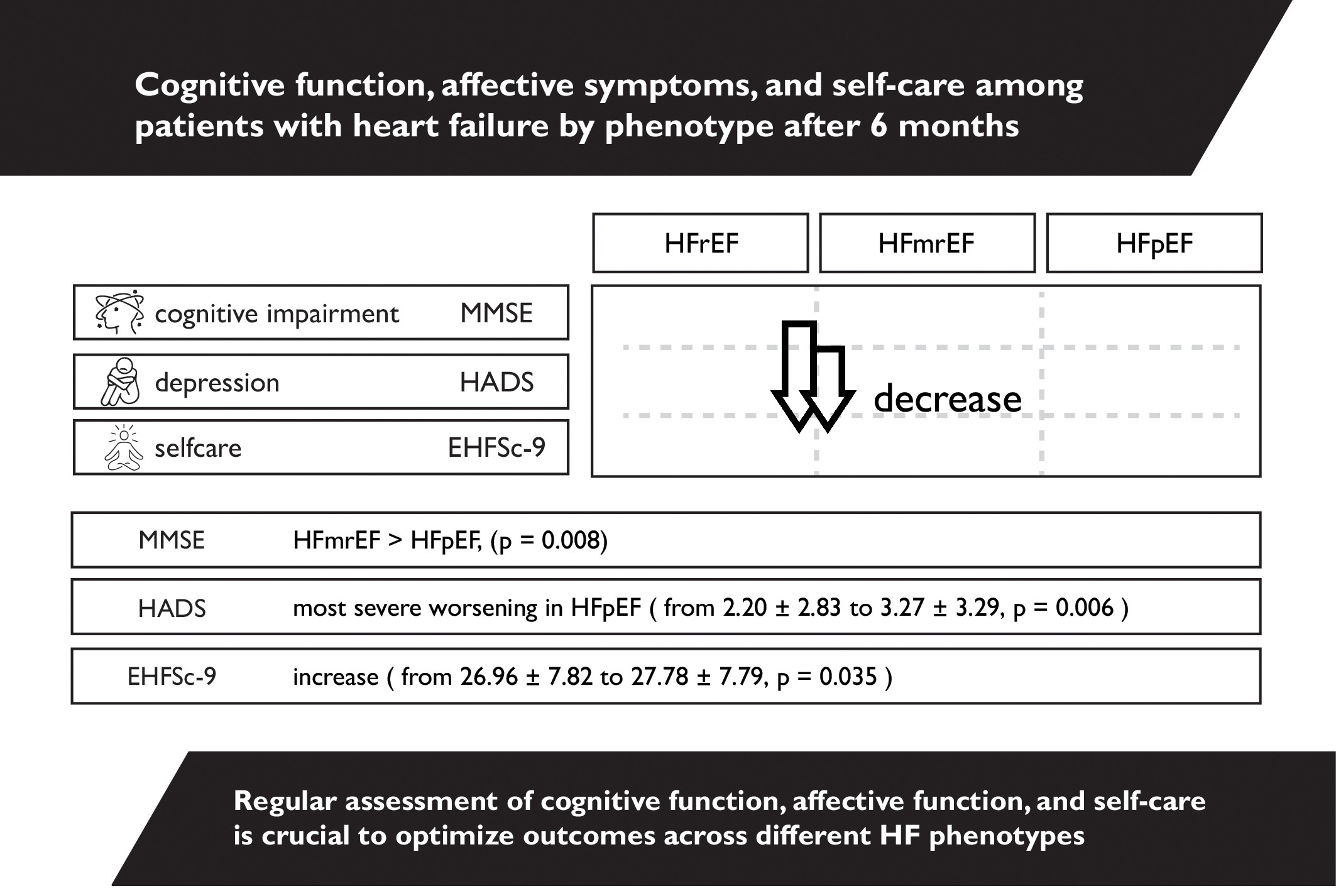
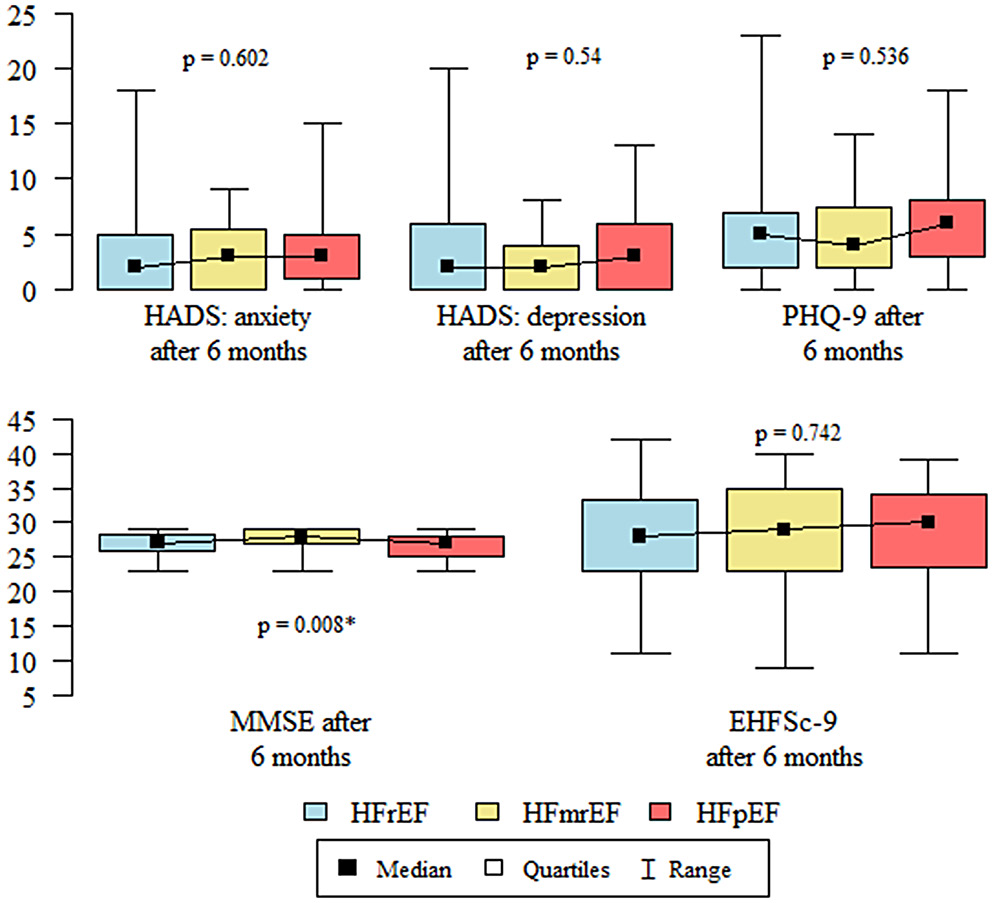
Results. Cognitive impairment indicated with the MMSE was less severe in patients with mildly-reduced HF (HFmrEF) compared to preserved EF (HFpEF) (MMSE median scores: 28 [interquartile range (IQR): 27–29] vs 27 [IQR: 25–28]; p = 0.008). The HADS showed that severity of depression worsened over 6 months, particularly in the HFpEF group (median scores increased from 1 [IQR: 0–4] to 3 [IQR: 0–6]; p = 0.006). Self-care ability declined in all groups as indicated in the increased EHFSc-9 (poorer self-care) median scores, which changed from 28 [IQR: 21–33] at baseline to 29 [IQR: 23–34] at 6 months (p = 0.035). Additionally, NT-proBNP parameters were higher in the HFrEF group (3437.7 pg/mL [IQR: 1336.33–6226.43) compared to both HFmrEF and HFpEF (2171.2 pg/mL [IQR: 806.65–4033.15] and 977.1 pg/mL [IQR: 576.9–3708.95, respectively, p = 0.001).
Conclusions. Patients with HF showed significant cognitive decline, increased depressive symptoms and reduced self-care over 6 months, with HFpEF patients exhibiting the most pronounced impairments. Differences in outcomes across HF phenotypes highlight the need for tailored diagnostic and therapeutic strategies to address cognitive and emotional challenges in this population.
Key words: anxiety, depression, self-care, heart failure, cognitive dysfunction
Background
Heart failure (HF) is a chronic condition that represents a growing global health burden, affecting millions of patients worldwide. With an aging population and advances in medical interventions that improve survival after acute cardiovascular events, the prevalence of HF is rising, especially among older adults.1 Heart failure, defined by the heart’s inability to pump blood efficiently, causes significant physical, cognitive and emotional impairments reducing patients’ quality of life (QoL) and increasing the burden on healthcare systems.2
Older adults with HF face an elevated risk for physical deconditioning, poor self-care and cognitive impairment (CI), irrespective of the ejection fraction (EF) subtype. Heart failure is categorized into 3 phenotypes of the EF: HF with reduced EF (HFrEF), mildly reduced EF (HFmrEF) and preserved EF (HFpEF).3 Cognitive impairment in HF has emerged as a major concern, as HF patients commonly exhibit deficits in memory, executive function, attention, and processing speed compared to those without HF symptoms.4 The underlying causes of CI in HF are multifactorial, involving reduced cerebral perfusion, vascular damage and the neurohormonal changes typical of chronic HF.5
Cognitive dysfunction appears to vary across HF phenotypes. For instance, HFpEF phenotype, which is more prevalent in older women, may be associated with milder CI compared to HFrEF.6 In contrast, HFmrEF patients experience distinct cognitive challenges, with some studies indicating a higher risk for hospitalization due to cognitive deficits and difficulty in self-care.7 These findings emphasize the need for clinicians to routinely assess cognitive function in HF patients, as unaddressed CI contributes to poorer self-care, increased hospital readmissions and higher mortality rates.8
Mental disorders, particularly depression and anxiety, are prevalent among patients with HF, and often exacerbate cognitive decline, contributing to poorer clinical outcomes. Depression in HF impairs cognition in terms of attention and executive function, which are critical for managing complex treatment regimens and self-care.9 Symptoms of anxiety can further complicate the clinical picture. Some studies suggest that mild anxiety may have a protective effect on cognitive function, whereas severe anxiety is associated with worsening cognitive outcomes.10
Effective self-care behaviors such as medication adherence, symptom monitoring and lifestyle adjustments are essential for managing HF and improving patients’ QoL.11 However, cognitive and emotional challenges often hinder self-care in HF patients. However, cognitive deficits in memory and executive functioning make it difficult for patients to follow complex medical regimens, while depression and anxiety reduce motivation and confidence in self-care abilities.12 This vicious cycle leads to worse health outcomes, including higher rates of hospitalization and mortality.13
Objectives
The aim of this study was to assess changes in affective symptoms, cognitive impairment and self-care behaviors in older adults with HF, categorized by HF phenotype, over a 6-month period.
Methods
Participants
This study was conducted 6 months after the 1st stage, participants were again re-examined. A total of 250 patients were enrolled in the 1st stage of the study. Of these, 77 did not participate in the 2nd stage due to inability to be contacted or other unspecified reasons. The 2nd stage included 162 participants, while 11 individuals died before the follow-up. To be eligible for inclusion in the 1st stage, participants had to meet the following criteria: age ≥60 years, a diagnosis of HF in accordance with the European Society of Cardiology (ESC) guidelines,14 a duration of HF of at least 6 months, hospitalization for acute HF, New York Heart Association (NYHA) functional class II–IV, and intact cognitive function, as assessed by the Mini-Mental State Examination (MMSE) with a score ≥24 points. The exclusion criteria in the 1st stage included NYHA class I, MMSE score <24 points, diagnosed and treated depressive disorder, and lack of consent to participate in the study.
Data collection
Patients were recruited from the Institute of Heart Diseases (Department of Cardiology) at University Hospital in Wrocław, Poland, between September 2022 and June 2023 for the 1st stage of the study. The 2nd stage of the study began 6 months after the 1st stage – starting in March 2023 and ending in December 2023. Patients were classified into 3 groups based on EF values: HFrEF: EF ≤40%, HFmrEF: EF 41–49% and HFpEF: EF ≥50%. The data were gathered during the hospitalization period after the successful treatment of acute decompensated HF, with clinical stability attained before discharge. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed.
Research instruments
Cognitive function was assessed using the MMSE, a tool developed by Folstein et al. in 1975.15 This test aimed to create a simple and rapid cognitive assessment tool that clinicians could easily use to identify disorders such as dementia or Alzheimer’s disease. The MMSE has become one of the most widely used screening tests worldwide for diagnosing cognitive problems. The test consists of simple questions and tasks assessing various aspects of cognitive functioning, including orientation in time and space, short-term memory, language abilities, attention, and mathematical skills. The maximum score on the MMSE is 30, and scores below 24 may indicate potential dementia.16 In this study, the Polish adaptation of MMSE developed by Stańczak was utilized.17
Depression and anxiety were measured using 2 instruments: The Hospital Anxiety and Depression Scale (HADS) and the Patient Health Questionnaire-9 (PHQ-9). The HADS was originally developed in 1983 by Zigmond and Snaith.18 It allows to quickly and easily assess levels of anxiety and depression in hospitalized patients, especially those with somatic illnesses, in whom physical symptoms could mask emotional disturbances. Many researchers have studied HADS data to determine cut-off points for anxiety and depression. Bjelland et al. identified a cut-off point of 8/21 for anxiety and depression through a review of numerous studies.19 This tool is extensively utilized in clinical and research settings to identify emotional disturbances. The HADS comprises 14 items, with 7 assessing anxiety and 7 assessing depression, each rated on a 4-point scale ranging from 0 to 3. The total score for each subscale ranges from 0 to 21, with higher scores indicating higher levels of anxiety or depression.20 The present study used the Polish adaptation of the HADS, validated by Mihalca and Pilecka.21
The PHQ-9 is a highly regarded self-report tool for assessing depressive symptoms. Developed by Kroenke and Spitzer, it has consistently demonstrated reliability and accuracy in measuring depression severity in a wide range of settings, from primary care to specialist medical practices.22 It includes 9 core questions and a supplementary question, providing a comprehensive assessment of depression. The respondent specifies the annoyance of the listed problems from “not annoyed at all” to “annoyed almost daily” in the past 2 weeks. Each question can be scored from 0 to 3 points, with a max of 27 points. Severe depression is indicated by a score greater than or equal to 20 points, moderate depression 15–19 points, moderate depression 10–14 points, and mild depression 5–9 points.23 The study used the Polish adaptation of the PHQ-9, which was validated by Kokoszka et al.24
The European Heart Failure Self-Care Behaviour Scale (EHFScB) was developed by Jaarsma et al. in 2003.11 The scale was created as part of research on self-care among patients with HF and is widely used in Europe and other regions. Its purpose is to support patients in better managing their health by assessing and monitoring their self-care behaviors. Subsequently, in 2009, the team led by Jaarsma revised the scale from a 12-item version to a 9-item scale, EHFScB-9, which can be used as an internally consistent and valid tool for measuring self-care behaviors related to HF.25 The 9-point scale consists of statements focusing on self-care skills in HF management. Five of these refer to specific self-care aspects, such as monitoring body weight, restricting fluids, adhering to a low-salt diet, taking prescribed medications, and engaging in physical activity. The remaining 4 assess symptoms (such as shortness of breath, extreme fatigue, lower limb edema, and significant weight gain over a week) that may indicate disease progression and warrant medical assistance. Responses are rated on a 5-point scale from 1 (“strongly agree”) to 5 (“strongly disagree”). The total score is calculated by summing the responses to all 9 statements, ranging from 9 to 45, where higher scores indicate lower self-care ability. A Polish adaptation of EHFSc-9 validated by Uchmanowicz et al. was used in the study.26
Ethical consideration
This study adhered to the principles of the Declaration of Helsinki and received approval from the Bioethics Committee of Wroclaw Medical University (approval No. KB-651/2022). Written informed consent was obtained from all participants before their inclusion in the study, and all patient data were anonymized to maintain confidentiality.
Statistical analyses
First, descriptive statistics were calculated for the quantitative variables, including mean, standard deviation (SD), median, quartiles, and minimum and maximum values. The analysis of qualitative variables was carried out by calculating the absolute frequencies and percentages of all values that these variables could assume. The comparison of qualitative variables between groups was conducted using the χ2 test of independence. If the assumptions of the χ2 test were not met, Yates’s correction was applied for 2 × 2 tables, while Fisher’s exact test was used for larger contingency tables. Quantitative variables across the 3 groups were compared using the Kruskal–Wallis test, followed by Dunn’s post hoc test if significant differences were identified. Correlations between quantitative variables were analyzed using Spearman’s correlation coefficient. Scatter plots were examined to verify the assumption of a monotonic relationship, and representative examples are provided in the shared data. The Wilcoxon signed-rank test was employed to assess differences between 2 repeated measurements. A significance level of 0.05 was adopted for the analysis. Each examined relationship was assessed independently rather than as part of a broader statistical inference encompassing all tests. Therefore, multiple comparison corrections were not applied, as each analysis was treated as an independent result. In this analysis, HF phenotype was considered a key confounding factor influencing the relationships between affective symptoms, cognitive impairment and self-care behaviors. While we recognize the presence of other potential confounding factors, they were not included in the scope of this study. This approach aligns with the primary aim of our research, which focuses on the role of HF phenotype in these associations. The analysis was performed with R software v. 4.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The demographic characteristics of the sample (n = 162) are summarized in Table 1. There were 76 patients with HFrEF, 55 patients with HFpEF and 31 patients with HFmrEF. The percentage of women was highest in the HFpEF group and lowest in the HFrEF group. The percentage of individuals from cities was highest in the HFmrEF group and lowest in the HFrEF group. At the beginning of the study, the percentage of individuals with normal weight was highest in the HFmrEF group and lowest in the HFpEF group, while the percentage of overweight individuals was highest in the HFrEF group and lowest in the HFmrEF group. The percentage of individuals with obesity was highest in the HFpEF group and lowest in the HFrEF group. Additionally, the percentage of individuals with central obesity was highest in the HFpEF group and lowest in the HFmrEF group. NT-proBNP levels were higher in the HFrEF group than in both the HFmrEF and HFpEF groups.
Table 2 shows the results of the questionnaires that were completed by people who died after the 1st stage of the study.
Statistical analysis showed that CI according to the MMSE after 6 months was significantly less severe in the HFmrEF group than in the HFpEF group (Table 3, Figure 1). The correlation between self-care behaviors and cognitive function and affective symptoms was examined within each HF phenotype group (Table 4).
Statistical analysis was performed, which compared the results of the questionnaires between the 1st and 2nd stages. The study showed that the level of self-care according to EHFSc-9 was significantly lower in stage II than in stage I. Depression, as measured with HADS, was significantly higher in stage II compared to stage I. Cognitive impairment according to the MMSE was significantly less severe in stage I than in stage II (Table 5, Figure 2).
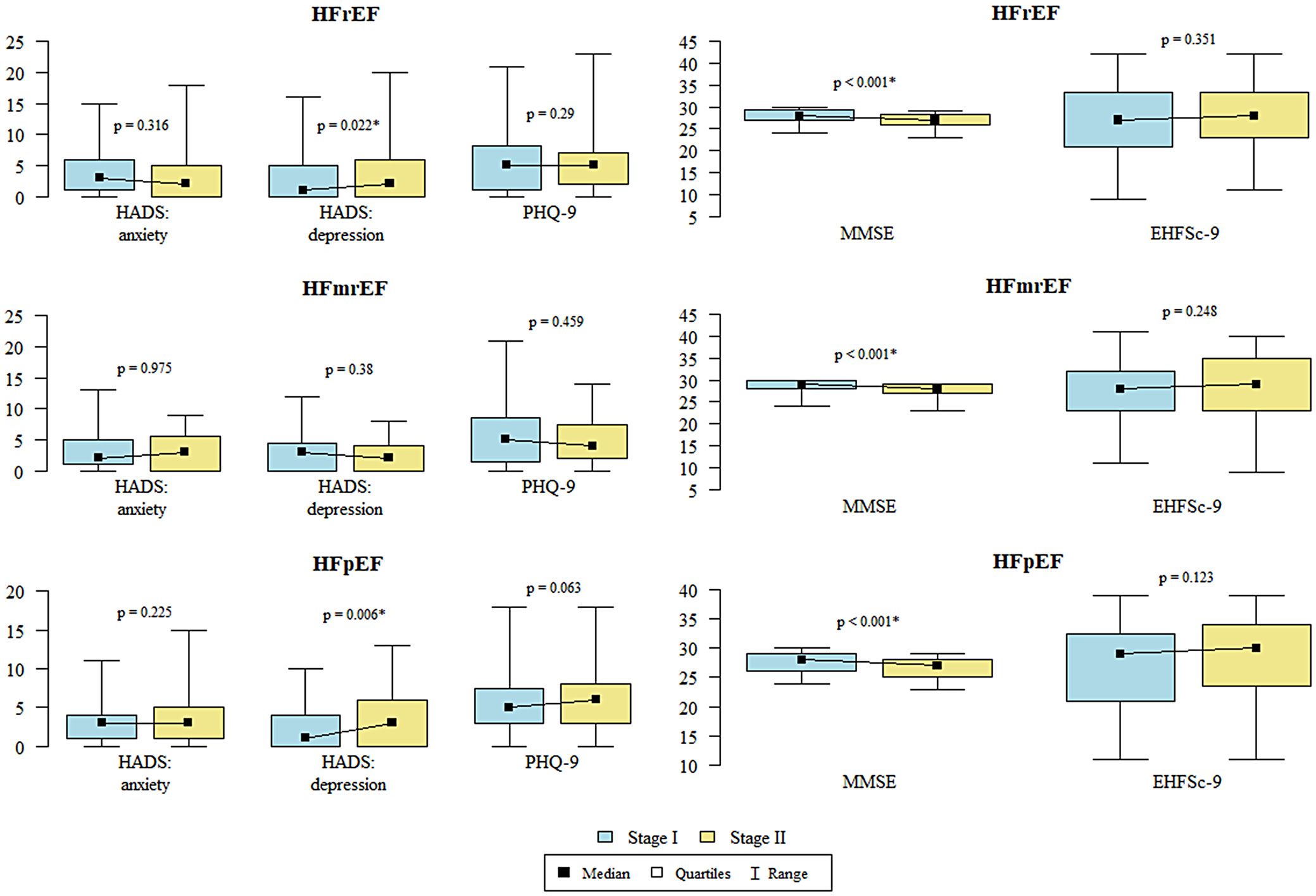
Statistical analysis for patients with HFrEF and HFpEF showed that the level of depression according to HADS was significantly higher in stage II than in stage I. Statistical analysis for patients with HfmrEF, HfrEF and HFpEF CI according to the MMSE was significantly less severe in stage I than in stage II (Table 6, Figure 3).
Discussion
The study presents a detailed analysis of HF subtypes (HFrEF, HFmrEF, HFpEF) in a diverse patient population, revealing significant differences in demographic, clinical and biomarker profiles. The predominance of women in the HFpEF group and the higher urban residency in HFmrEF suggest potential sociodemographic influences on disease presentation. Body mass index and central obesity differences across groups underscore the metabolic effects of HF phenotypes. Notably, the higher NT-proBNP in patients with HFrEF phenotype as compared to HFmrEF and HFpEF suggest the need of more aggressive management strategies in these patients.
These findings are in line with other major studies, such as the Framingham Heart Study (FHS) and the Multi-Ethnic Study of Atherosclerosis (MESA).28 Both studies reported that the lifetime risk of HFpEF was higher than that of HFrEF, particularly among women. The FHS cohort reported a rise in lifetime risk across both sexes, with notable variations by race and ethnicity in MESA and the Cardiovascular Health Study (CHS). The higher prevalence of HFpEF and its associated risk factors in the above study emphasizes the need for targeted interventions across different demographic groups.6, 27
This study’s findings align with existing research, confirming that CI is a significant issue in patients with HF. The observed cognitive decline mirrors findings from broader studies that indicate a high prevalence of CI among HF patients, with rates ranging from 10% to 79%. This cognitive decline is linked to poorer self-care, increased hospitalization and higher mortality rates. Studies such as the one by Kuipers et al. further highlight the importance of proactive CI screening and management in HF patients to enhance overall outcomes.8 The implications of these findings are substantial. Given the demonstrated association between HF and CI, especially in HFpEF, it is crucial to incorporate regular cognitive assessments into standard care for HF patients. This approach would enable more personalized treatment strategies addressing both cardiovascular and cognitive needs. Additionally, as highlighted in the broader literature, the varied risks associated with different HF subtypes underscore the need for tailored interventions to mitigate risks and improve long-term outcomes.
Medication patterns, particularly the higher use of diuretics and mineralocorticoid receptor antagonists (MRA) in HFrEF, reflect the necessity for tailored treatment approaches for each HF subtype. These findings are consistent with established guidelines that emphasize the critical role of these medications in managing HFrEF. While diuretics are essential for alleviating congestion and reducing hospitalizations, their use must be carefully balanced with other guideline-directed medical therapies (GDMT) to avoid complications such as hypotension and renal dysfunction. This tailored approach highlights that, while diuretics are not disease-modifying, they play a crucial role in symptom management and achieving euvolemia.28, 29, 30 It emphasizes the importance of personalized care in HFrEF, where severity of symptoms and comorbidities demand a careful pharmacotherapy balance. Our findings highlight the need for vigilance in managing these complex cases, ensuring that life-saving treatments are maximized while minimizing adverse effects, in line with current clinical guidelines and evidence-based practices.
Cognitive impairment was more pronounced in HFpEF, which can affect how these patient manage their condition and present self-care behaviors. This finding emphasizes the importance of cognitive assessments as part of regular care for HF patients. Research by Uchmanowicz et al. supports this highlighting connection between frailty syndrome and cognitive decline in patients with HF condition. The combined presence of frailty and cognitive impairment significantly increases the risk of adverse outcomes, such as higher mortality, increased hospital readmissions and poorer QoL. These insights point to the need for comprehensive care strategies that address both the cognitive and physical challenges faced by older and frail HF patients.12
Finally, the mortality analysis revealed that patients who passed away after the initial stage had poorer scores in cognitive and depression assessments. This finding highlight potential for using risk stratification and management strategies, focusing on improving mental health and cognitive function as part of comprehensive HF care. Our study’s findings that diminished cognitive functioning and more severe depression scores are associated with increased mortality in HF patients align closely with the broader literature. For example, Gathright et al. conducted a meta-analysis demonstrating that depression is a significant predictor of all-cause mortality in HF patients, particularly among older adults and during shorter follow-up periods.9 Similarly, Rutledge et al. found a strong link between depression and increased mortality in HF.13 Moreover, studies emphasize the growing burden of HF, where mental health plays a crucial role in managing this chronic condition.2, 31, 32
Given the evidence from these studies, it is essential to integrate comprehensive mental health care into the routine HF management to earlier identify and treat at-risk individuals, improving survival rates and QoL. Consistent evidence from various studies and meta-analyses further highlights the need for a multidisciplinary approach that addresses both the physical and psychological dimensions of HF.
Overall, this study underscores the heterogeneity within HF populations and the importance of personalized treatment approaches to account for demographic, clinical and psychosocial factors to achieve the best outcomes for patients.
Limitations
Despite its strengths, this study has several limitations. First, its observational design makes it difficult to establish causal relationships between the identified factors and HF outcomes. Additionally, the sample size may have been insufficient to capture the full spectrum of variability within each HF subtype, particularly when examining less common comorbidities or demographic subgroups. Furthermore, the reliance on self-reported data for certain variables, such as cognitive function and depression, could introduce bias or inaccuracies, particularly if patients underreported or overreported their symptoms. Additionally, while the study considered a range of demographic and clinical variables, other potentially relevant factors, such as socioeconomic status, access to care and lifestyle factors, were not comprehensively analyzed. Finally, the follow-up period, although adequate for short-term outcomes, may not capture long-term trends and outcomes, particularly regarding the progression of cognitive impairment and its impact on mortality. Future studies with extended follow-up periods and larger, more diverse populations would help mitigate these limitations and offer a more comprehensive understanding of HF subtypes.
Practical implications
The findings of this study carry several practical implications for the management of patients with HF. The diversity in clinical profiles across HF subtypes suggests that a personalized approach to treatment is essential. Clinicians should consider demographic factors, such as gender and residence, alongside clinical indicators like BMI and NT-proBNP levels, to tailor treatment strategies effectively. Moreover, the significant cognitive impairment observed, particularly in HFpEF patients, underscores the necessity of incorporating cognitive and psychological assessments into routine care. This approach could enhance patient adherence to treatment and improve overall health outcomes. Finally, the higher mortality rates associated with worse cognitive and depression scores underline the importance of addressing mental health in HF management. Overall, these implications underscore the necessity of a holistic and individualized approach to treating HF, which may enhance patient outcomes and optimize the utilization of healthcare resources.
Conclusions
This study highlights significant differences among HF phenotypes in cognitive function, depression and self-care behaviors. Patients with HFpEF exhibited the most severe cognitive impairment and progressive depressive symptoms, while NT-proBNP levels were highest in HFrEF, highlighting the need for more intensive management. The findings underscore the importance of integrating routine cognitive and psychological assessments into HF care and developing phenotype-specific therapeutic strategies to optimize patient outcomes. Tailored interventions that address the specific challenges of each HF subtype, particularly cognitive deficits and depression, are essential for enhancing long-term health outcomes and QoL.
Data availability statement
The dataset used in this study is publicly available at Zenodo: https://doi.org/10.5281/zenodo.14996762.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.