Abstract
Background. Evidence regarding the optimal timing of peritoneal dialysis catheter (PDC) removal in renal graft recipients is limited. While some centers opt for removal during the transplant procedure, others defer catheter removal to various time points post-transplantation.
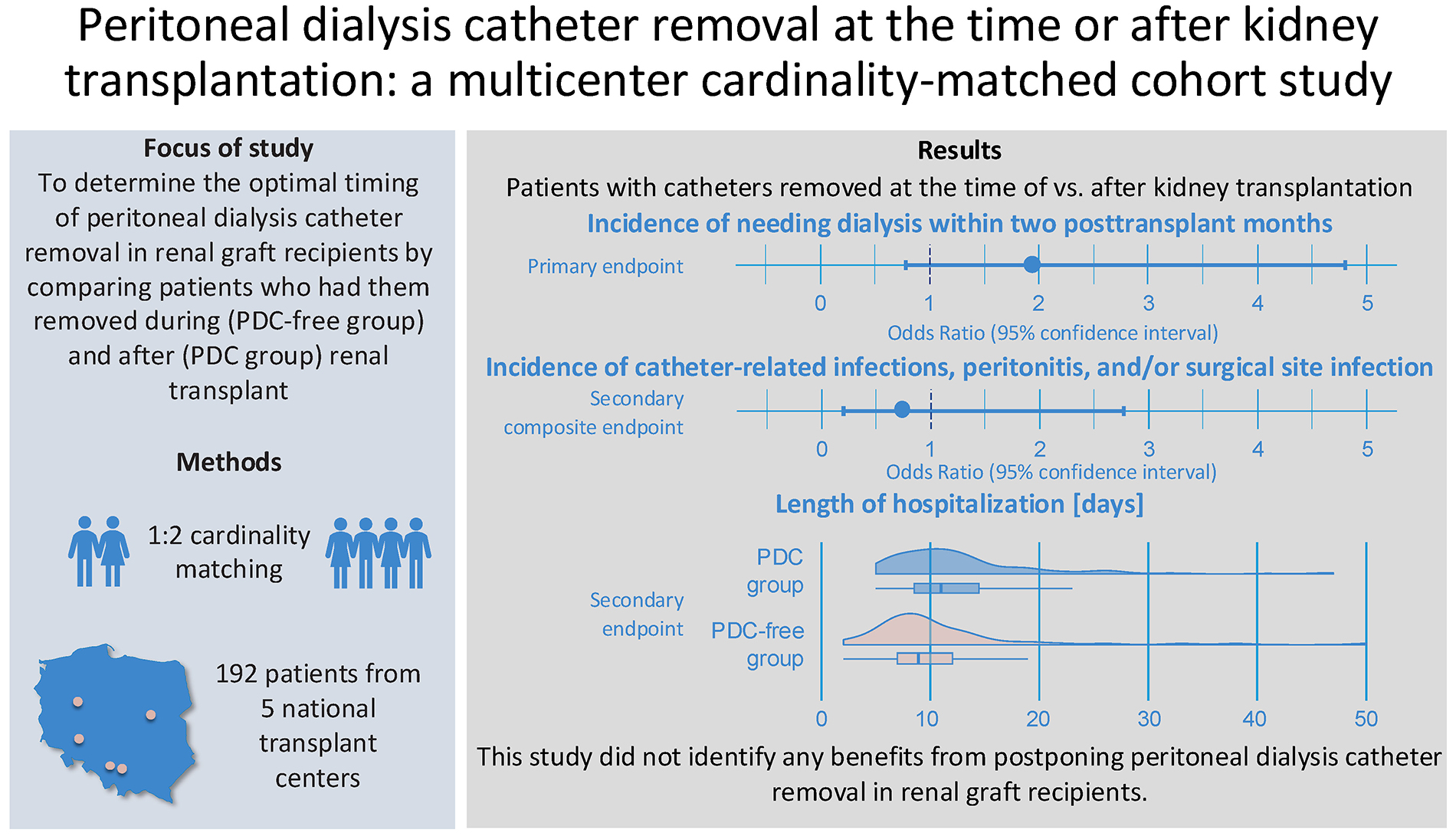
Objectives. In this multicenter cardinality-matched cohort study, we aimed to determine the optimal timing of PDC removal in patients undergoing kidney transplantation.
Materials and methods. Data from 324 patients were collected across 5 centers. We compared patients who had catheters removed during renal transplant (the PDC-free group) with those who had them removed after the procedure (the PDC group), matched 1:2 by age, sex, body mass index (BMI), living, and extended criteria donor statuses. We evaluated: 1) the need for dialysis within 2 post-transplant months, 2) a composite endpoint of catheter-related infection, peritonitis and/or surgical site infection, and 3) the length of hospitalization.
Results. After cardinality matching, the groups were well-balanced across all matching covariates. Postoperative dialysis was required in 14% of patients, with no statistically significant difference observed between the PDC-free and PDC groups (19% vs 12%; odds ratio (OR) = 1.94; 95% confidence interval (95% CI): 0.78–4.81; p = 0.152). Of the 14 patients in the PDC group who required dialysis postoperatively, only 3 were managed with peritoneal dialysis. No statistically significant difference was noted for the composite endpoint (8.6% vs 6.2%; OR = 0.74; 95% CI: 0.20–2.77; p = 0.656). Hospitalization was significantly longer in patients from the PDC group (median [interquartile range (IQR)]: 11 [9–15] vs 9 [7–12]; BM = −3.036; p = 0.003).
Conclusions. This study did not demonstrate any benefits associated with delaying PDC removal in renal graft recipients. On the contrary, postponing removal was linked to prolonged hospitalization.
Key words: kidney transplantation, peritoneal dialysis, delayed graft function, catheter-related infections
Background
The perioperative care of peritoneal dialysis patients undergoing renal transplantation involves addressing various challenges across the preoperative, intraoperative and postoperative phases.1 These include but are not limited to optimizing dialysis, controlling the increased risk of catheter-related infections, including peritonitis, balancing fluid volume and nutritional statuses, monitoring surgical wound healing, ensuring rejection surveillance, and establishing alternative acute or chronic renal replacement therapy in case of graft failure or loss.2 Another dilemma that transplant surgeons face is related to providing peritoneal dialysis catheter (PDC) management that is most beneficial for the renal recipient. The problem can be summarized by the clinical question of whether PDCs should be left intact or removed during the transplant procedure.3
Unfortunately, there is currently no consensus or robust evidence to guide transplant teams on the optimal timing for PDC removal in renal graft recipients.3, 4 Different centers and individual transplant surgeons follow various policies. These include routine removal of the catheters at the time of transplant3, 4, 5, 6, 7, 8 or postponing it until various postoperative time points.9, 10 The European Best Practice Guidelines for peritoneal dialysis suggest they can be left intact for around 3–4 months following transplantation even if good graft function is observed.11 However, these guidelines, published in 2005, have never been updated (also by other peritoneal dialysis expert groups or societies) and faced criticism due to their methodological constraints.12
A recent systematic review and meta-analysis concerning 8 non-randomized studies of interventions did not provide a definitive answer to the question about optimal catheter removal in renal graft recipients due to the high risk of bias of the included studies.13 It showed that several factors should be taken into account when planning perioperative and post-transplant catheter management, i.e., the risk of early graft dysfunction requiring renal replacement therapy (including acute and chronic dialysis), the risk of infectious or other catheter-related complications, as well as the patient’s preferences.
According to Gardezi et al.,14 patients receiving peritoneal dialysis are more likely to undergo kidney transplantation compared to those on hemodialysis. Similar trends are also observed in Poland where around 4% (4.24% in 2021 and 4.17% in 2022) of dialyzed patients are treated with peritoneal dialysis.15 Taking that into account, there is an increasing need to provide objective evidence to help clinicians and patients plan optimal dialysis catheter management to ensure the best possible transplant outcome, including quality of life, and potentially reduce the number of subsequent hospitalizations and costs.
Objectives
The objective of this retrospective cohort study was to determine the optimal timing of PDC removal in renal graft recipients by comparing patients with catheters removed at the time of and after kidney transplantation, concerning the incidence of requiring dialysis after the procedure, infectious complications and the length of hospitalization.
Materials and methods
Study design
We conducted a multicenter retrospective cardinality-matched cohort study in peritoneal dialysis patients undergoing kidney transplantation. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) and Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) 2021 guidelines.16, 17
Ethical approval
Ethical approval and informed consent collection were waived by the local Ethics Committee of the Medical University of Warsaw, Poland (decision No. AKBE/26/2021) due to the retrospective character of this study. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Setting and participants
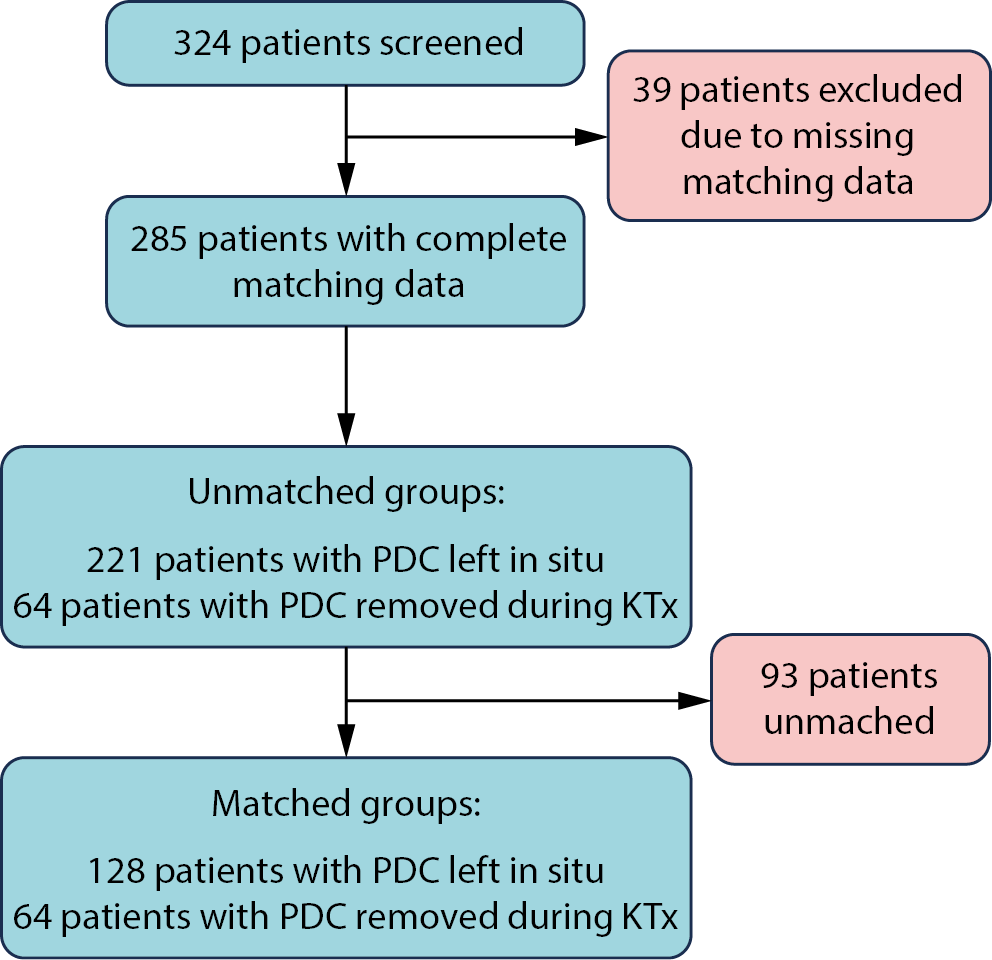
Retrospective data concerning adult (≥18 years old) renal graft recipients previously managed with peritoneal dialysis were collected from patient’s medical records across 5 kidney transplant centers in Poland. Patients transplanted between 2010 and 2021 were considered eligible for inclusion, ensuring at least 1 year of follow-up. We excluded patients with PDCs removed more than 1 day before the transplant procedure, those who underwent multiorgan transplantation, or individuals with Bricker ileal conduit urinary diversion. Patients with unknown group assignment (no information on whether the PDC was removed at the time or after kidney transplantation) or with missing matching variables were also excluded from the final analyses.
Both deceased donor (all after brain death) and living donor renal transplant procedures were included in the study. The PDC removal timing was at the discretion of the transplant surgeon or other treating physician. Some of them preferred removing the catheters at the time of transplantation, while others postponed it. The achievement of stable graft function most commonly defined the time of delayed removal.
Variables
Medical records from transplant wards and outpatient clinics were searched to collect recipients’ and donors’ age, sex, body mass index (BMI), subjects’ cumulative dialysis vintage (in months), and number of episodes of peritonitis before and after transplantation. Donor characteristics, including extended criteria and living donor status, were identified. Renal graft’s cold ischemia, vascular anastomosis and operative times (all in minutes), graft storage modality (including simple cold storage, hypothermic machine perfusion and their combination), and PDC placement site were extracted. Information about the use of central line catheters during surgery, surgical drainage placement, as well as length of hospitalization and surgical drainage use (both in days) were collected. Data concerning intraoperative peritoneum breaches, ascites, urinary leakage, urinary tract infections, catheter-related infections, delayed graft function (defined as the need for dialysis within 7 post-transplant days), types of dialysis used following surgery, and peritoneal dialysis removal time after transplantation (in days), and 1-year graft survival were evaluated.
Study endpoints
The primary endpoint was the incidence of needing dialysis within 2 post-transplant months.13 Secondary objectives included a composite endpoint defined as the incidence of catheter-related infection, peritonitis and/or surgical site infection, as well as the length of hospitalization.
Cardinality matching
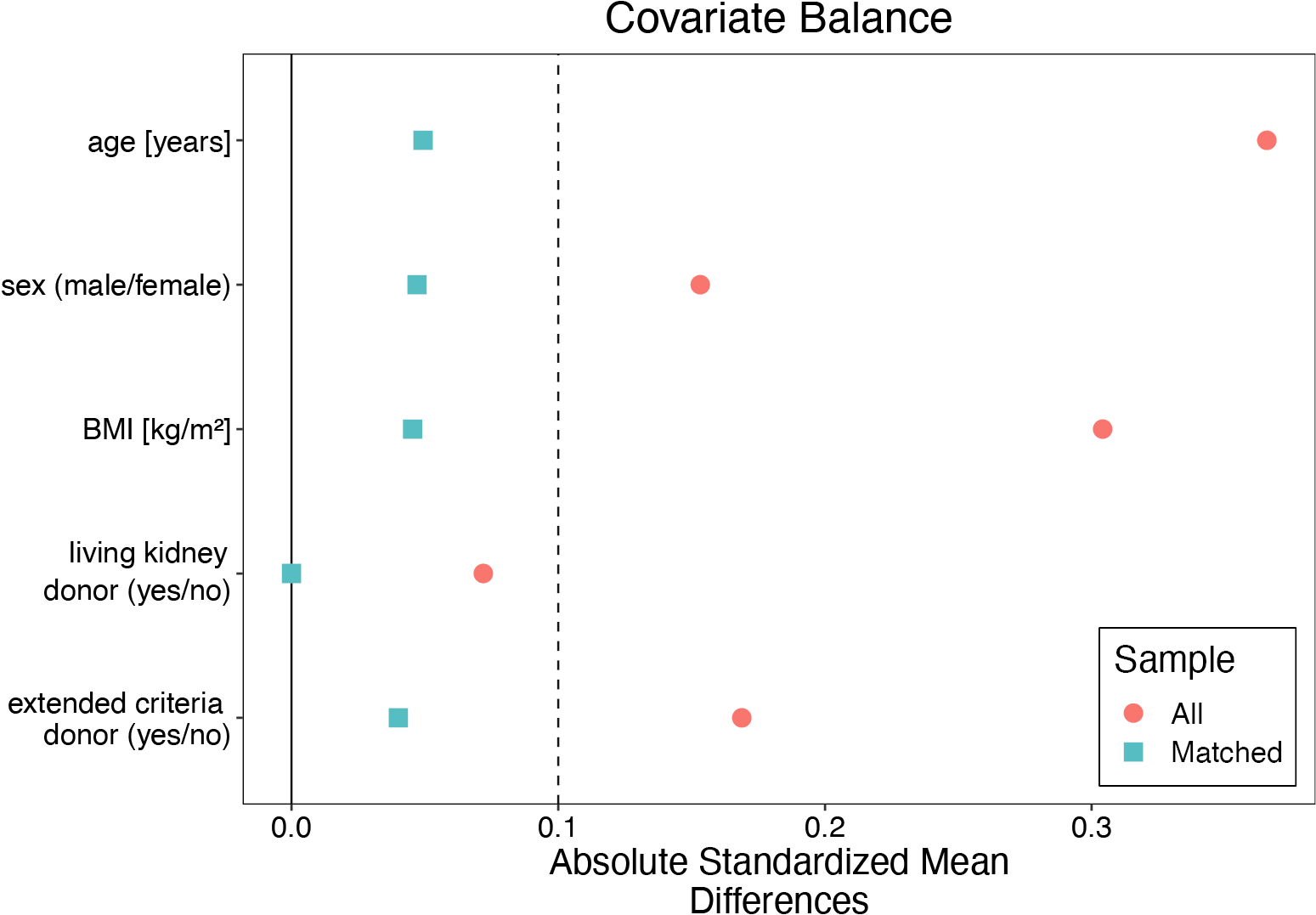
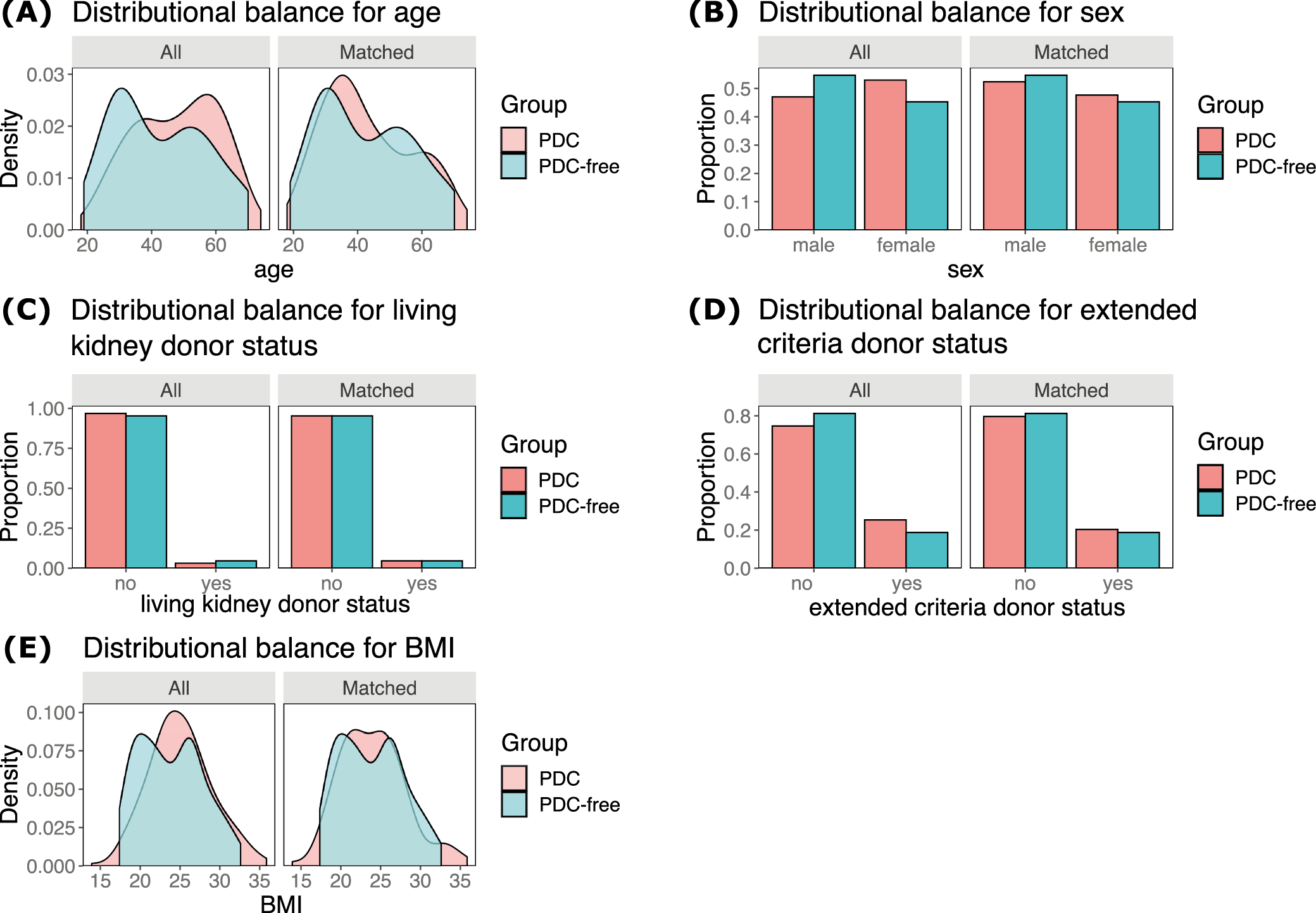
All patients were assigned to the group of recipients whose PDCs were removed at the time of renal transplantation (the PDC-free group) or after the procedure (the PDC group). Cardinality matching18 was used to match patients 1:2 by 5 covariates, i.e., kidney recipient’s age, sex, BMI, living, and extended criteria donor statuses. These factors were selected based on the discussion and consensus among the co-authors, following the evaluation of a web of causation and literature search (to identify factors affecting both the peritoneal dialysis removal and the need for dialysis early after transplantation). Cardinality matching uses advanced programming techniques to find the largest matched sample balanced by prespecified covariates without relying on, and thus overcoming some limitations of, the propensity scores or coarsened covariate values.19, 20, 21 The matching was performed using the MatchIt package22 in R with the optimization performed by the HiGHs optimization solver.23 An imbalance between the groups was considered when absolute mean differences were greater than 0.1.
Study size
Assumptions for sample size calculation were based on the study by Kwong et al.24 and the recent meta-analysis of observational studies.13 Setting α error at 0.05, β error at 0.2, the allocation ratio 1:2 and assuming the proportion for the primary endpoint at 0.19 in the PDC and 0.05 in the PDC-free group, we calculated the required sample size of 186 (62 subjects in the PDC and 124 in the PDC-free group). The calculations were performed using a priori sample size calculation for Fisher’s exact test in G*Power v. 3.1.9.7 (Heinrich Heine University, Düsseldorf, Germany, 2020).25
Statistical analyses
Continuous variables were summarized using means with standard deviations (SDs) or medians with interquartile ranges (IQRs), depending on whether the normal distribution was determined using the QQ plot, histogram and the Lilliefors test assessment. The Lilliefors test was given priority over other assessments when evaluating the distribution of continuous data. Categorical covariates were expressed as the number of observations and percentages. No imputation was used to replace missing data (missing data were deleted pairwise). Differences between the groups for categorical variables were evaluated using Fisher’s exact (when an expected value was less than 5) or χ2 tests of independence, as appropriate. For continuous variables, the permuted Brunner–Munzel test was used.
In the matched cohort, the average marginal treatment effect was evaluated with G-computation using robust confidence intervals.26 For the primary and secondary composite endpoints, logistic regression models with the matching variables as covariates, and matching weights included, were created. The results were presented using odds ratios (ORs) and robust 95% confidence intervals (95% CIs). Assumptions of the logistic regression models were verified using the Box–Tidwell test, Variance Inflation Factor evaluation and Cook’s distance analysis. The Bonferroni correction was applied to account for multiple comparisons. A two-sided p-value less than 0.0167 (to account for the multiple tests performed) was considered statistically significant. All calculations, statistical tests and visualizations were performed using the MatchIt,22 HiGHS,23 cobalt,27 ggplot2,28 easyalluvial,29 tableone,30 and marginaleffects31 packages in R v. 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Sample characteristics
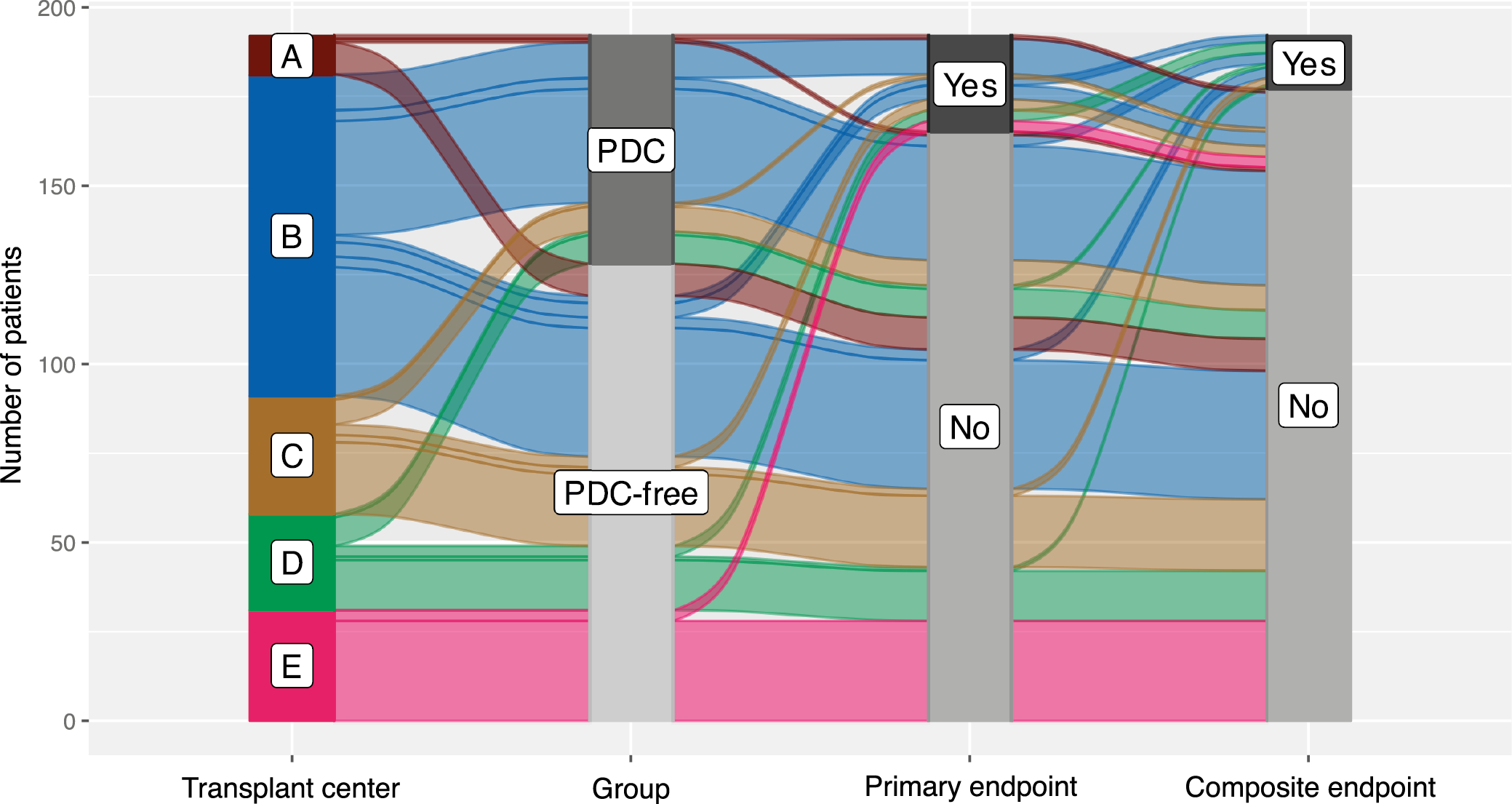
We collected data from 324 patients from 5 transplant centers. After excluding those with missing data for the matching or grouping variables, 324 were considered eligible for inclusion. In the final analysis, 64 patients from the PDC-free group were matched 1:2 with 128 patients assigned to the PDC group (a total sample size of 192). The flow of patients in the study is summarized in Figure 1.
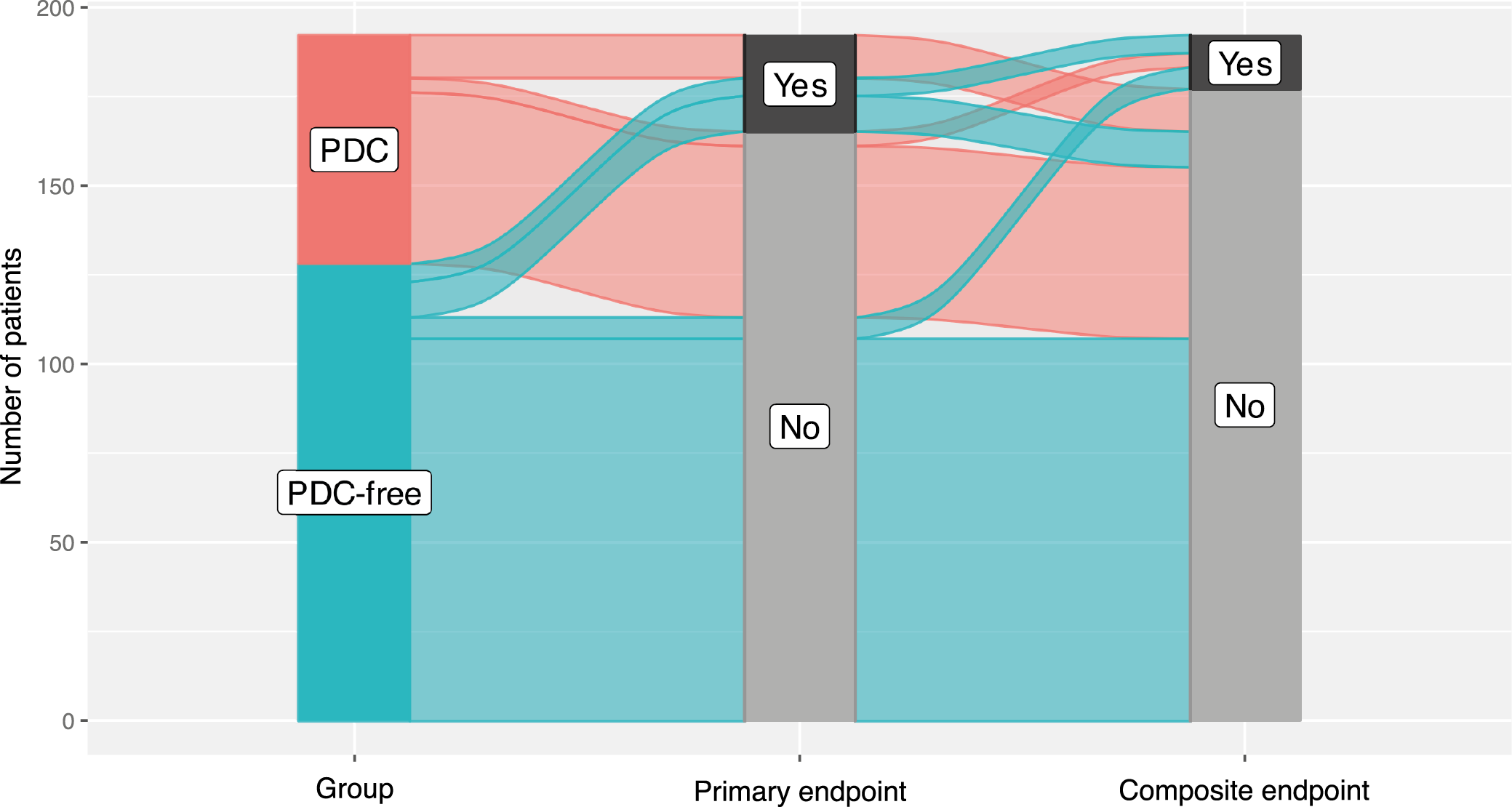
After the cardinality matching was conducted, 2 groups balanced (absolute standardized mean difference (SMD) <0.1) in terms of all matching variables were established (Figure 2, Figure 3). The studied population consisted of 47% of females (48% in the subgroup of kidney recipients who had their PDCs removed after and 45% in those with catheters removed at the time of surgery). The operative time was longer in patients with catheters removed at the time of surgery (median [IQR]: 150 [130–181] vs 130 [105–156] min; BM = 3.403, p = 0.002). The peritoneum was left intact following the procedure in 66% (84/128) of subjects from the PDC group and 64% (41/64) of those from the PDC-free group (χ21 = 0.046, p = 0.830). A central venous catheter was inserted more often in the PDC-free group (90% vs 53%; χ21 = 25.991, p < 0.001). Surgical drainage was utilized in 97% of subjects (98% in the PDC and 95% in the PDC-free group; p = 0.336). The median [IQR] time to PDC removal in the PDC group was 64 [42–97] days (the shortest time was 6 days and the longest one 243). Key baseline characteristics of the studied population are summarized in Table 1, while operative and postoperative characteristics are presented in Table 2.
Primary and secondary endpoints
The primary endpoint, defined as the need for dialysis within 2 post-transplant months was observed in 19% of patients from the PDC-free and 12% of patients from the PDC group, with no statistically significant difference noted between the groups (OR = 1.94; 95% CI: 0.78 – 4.81; p = 0.152, Table 3). Only 3 out of 14 subjects with PDCs available underwent peritoneal dialysis (Table 2). Similarly, no clinically and statistically significant difference was identified for the secondary composite endpoint of the incidence of catheter-related infections, peritonitis and/or surgical site infections. It was detected in 6.2% of patients with PDCs removed at the time of transplant and in 8.6% of recipients with catheters removed after the surgery (OR = 0.74; 95% CI: 0.20 – 2.77; p = 0.656, Table 4). These outcomes are presented graphically in Figure 4 and Figure 5. No significant differences were also identified when catheter-related infections other than peritonitis, peritonitis and surgical site infections were evaluated separately (Table 2).
The length of hospitalization was statistically significantly shorter in the PDC-free group (median [IQR], 9 [7–12] vs 11 [9–15] days; BM = −3.036, p = 0.003).
Discussion
In this pragmatic cardinality-matched cohort study, we showed that there was no statistically significant difference in the incidence of needing dialysis within the first 2 post-transplant months between patients whose PDCs were left in situ during kidney transplantation and those whose catheters were removed at the time of the procedure. Accordingly, we could conclude that the current decision-making based solely on the surgeon’s discretion is not optimal and fails to predict which patients could potentially benefit from postponing the catheters’ removal. Therefore, we seek to determine more objective factors to guide the medical practice on the optimal peritransplant management of peritoneal dialysis patients.
Interestingly, in our study, more patients had their Tenckhoff catheters left in place than removed at the time of transplantation. A similar proportion was presented in a study by Warren et al,.5 who analyzed data from 2 centers in the UK. Unfortunately, it is impossible to retrospectively evaluate why this approach was preferred. What could give some hints is the longer cold ischemia time in the PDC group (Table 2) which, however, was not associated with a higher risk of needing dialysis early after transplantation. Nevertheless, feasible is the evaluation of whether attempts of peritoneal dialysis following transplantation were successful. The results are not very promising. Only 3 out of 14 patients (3 out of 27 in the unmatched cohort, Table 2) requiring dialysis underwent peritoneal dialysis despite having Tenckhoff catheters left intact during their transplant procedures. Reports based on large databases prove that there is potential to use peritoneal dialysis more frequently. In France, according to a report by the Biomedicine Agency from 2013, only 5.1% of patients with failed allografts receive peritoneal dialysis32 while in the USA this figure was at 16% (data from the United States Renal Data System).33 Furthermore, the patients’ preferences are also important to consider when selecting between various dialysis modalities following graft failure. These are highly individualized and usually depend on various factors, including, i.a., individual circumstances, medical history, lifestyle considerations, experience, expectations, and perceptions.34 Nevertheless, this is also influenced by the actual availability of both dialysis modalities and whether patients are provided by their healthcare providers or insurers with the option to choose between them.
Assuming that both the clinical team and the patient would consider having a PDC left in place during the transplant procedure, a question remains: How to predict the risk of needing dialysis after the procedure? There are 2 main different scenarios when dialysis might be needed shortly after kidney transplantation, i.e., in case of delayed graft function or primary non-function/early graft loss. Unfortunately, the process of restarting any type of dialysis after kidney transplant failure is not well addressed in clinical guidelines.35 In the meta-analysis of 8 non-randomized studies of intervention from 2022, the pooled prevalence of needing dialysis early after transplantation for any reason was 15.2% (95% CI: 11.1–20.3%) in patients with PDCs removed at the time of transplantation and 8.6% (95% CI: 2.7–24%) in the group with Tenckhoff catheters removed after surgery.13 A similar trend was noted in the current study (19% vs 12%), despite cold ischemia time being longer in the PDC group (Table 1). Over a decade ago, a good and practical concept of using web-based calculators to predict delayed graft function was proposed by Irish et al.36 A similar tool could be developed to help predict primary non-function. Considering the better availability of high-volume real-world data and recent advancements in calculation techniques, we recommend revisiting this idea and creating newer validated predictive models. Bearing in mind the high variability in population characteristics among various continents and countries, it would be appropriate to include as diverse and big a population as possible.
Another dilemma that might appear is whether it is safe to proceed with peritoneal dialysis shortly after kidney transplantation. Potentially high risk of peritoneal breaches might be concerning to surgeons. In our study, despite grafts being implanted extraperitoneally, the peritoneum was assessed by surgeons to be intact in around 65% of cases, without any notable differences between the evaluated groups. According to Issa and Lakhani,37 a compromised peritoneum is an indication to remove the PDC during a renal transplant. Nevertheless, in the absence of contraindications, the evidence about peritoneal dialysis initiation shortly after transplantation is compelling and suggests that such treatment is safe.38 Furthermore, a randomized controlled trial (RCT) proved that it is feasible and safe to use PDCs even immediately after insertion.39 In that study, no relevant differences were noted when patients with urgent and delayed utilization groups were compared regarding catheter-related complications and catheter survival within 1 year following the insertion. Another doubt might be related to the concern of potential infectious complications, including catheter-related infections and peritonitis.4, 5 The results of our study did not show such trends which is consistent with other reports.13 We did not identify clinically relevant increased rates of peritonitis shortly after transplantation among patients with catheters left in situ. However, we were not able to evaluate long-term results.
Unfortunately, there is still insufficient evidence available to compare the 2 approaches to peritransplant PDC management in terms of the patient’s outcomes, including overall graft survival and quality of life, as well as short and long-term costs. We noticed that the removal of PDCs during surgery prolongs the procedures by around 20 min. The costs to the healthcare system can go up when the catheters are left intact due to the need for subsequent hospitalizations and procedures required to eventually remove them. Costs and additional risks might be also related to potential complications associated with such interventions, including those related to the use of general anesthesia that might be used in some cases. Unfortunately, the retrospective nature of data collection and the variability in local treatment practices limited our ability to evaluate such complications in detail. However, what was identified in our study is that patients with catheters left in situ stayed statistically significantly longer in the hospital after their transplant procedures. The hospitalization prolongation was about 2 days, which for patients on immunosuppression may be considered clinically significant due to increased risk of hospital-acquired infections. This outcome has an uncertain origin and, to our knowledge, was not described before. We hypothesize that it might be related to the desire to observe such patients more closely for signs of potential catheter-related infections early after transplantation. It could also be related to delayed graft function or the need for dialysis before the initial discharge. However, delayed graft function was more frequent in the PDC-free group (17% vs 9.4%, Table 2). It is also possible that it was due to some other unidentified factors.
With an increasing number of patients using peritoneal dialysis worldwide, the dilemma of Tenckhoff catheter removal in such patients might become more frequent and put a more noticeable burden on healthcare systems in the future. What is worth pointing out is that based on various, often negative experiences, some authors declared to switch to a routine of removing PDCs during kidney transplantation.3, 4, 5
Limitations
The present study study is limited by its retrospective design. This issue was mitigated by the use of cardinality matching, which yielded satisfactory results in terms of getting balanced groups. Nevertheless, the risk of unmeasured confounding persists.19 Despite including patients from 5 transplant centers and setting a broad data collection period, the sample size was not very big. This proves that although an RCT would be the best method to answer the studied dilemma, it requires a large number of participating sites and countries and/or a long enrollment period which would significantly increase its potential costs. In contrast, no external funding was needed to complete this matched cohort study which to some extent emulates a true RCT.19, 40 Moreover, cardinality matching is thought to bring some advantages in the setting of a small sample size when compared to other observational data analysis methods.41 Our data can be used to power a prospective study. What should be considered in future research that could not be evaluated in ours are patient-reported outcomes, including quality of life and satisfaction assessments.
Conclusions
We did not identify any significant benefits from postponing PDC removal in patients undergoing kidney transplantation. Neither clinically nor statistically significant differences were noted for the need for dialysis within 2 post-transplant months when patients with catheters removed at the time and after the procedure were compared. Postponed catheter removal was not associated with an increased rate of infectious complications. However, it was associated with prolonged hospitalization. An individual approach based on a detailed risk-benefit assessment and patient preferences should be taken into account when planning PDC management in renal graft recipients.
Data Availability Statement
The datasetssupporting the findings of the current study are openly available in the Open Science Framework (OSF) repository at DOI 10.17605/OSF.IO/8A4RW.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.