Abstract
Background. Elevated intracranial pressure (ICP) significantly worsens neurological outcomes and mortality rates in patients with traumatic brain injury (TBI). Hypertonic saline (HTS), a hyperosmolar treatment, controls elevated ICP in TBI patients. However, there is still debate regarding the efficacy of HTS in managing TBI.
Objectives. To assess the effectiveness of HTS in lowering elevated ICP in TBI patients with TBI.
Materials and methods. A systematic search was conducted using 4 electronic databases (PubMed, Embase, Scopus, and Cochrane Library) to select relevant articles published in peer-reviewed journals. The risk ratio (RR) and mean difference (MD) were calculated, along with their 95% confidence intervals (95% CIs). Heterogeneity was assessed using Cochrane Q, I2 statistics and p-value. RevMan 5.4 was used.
Results. The current meta-analysis included 965 TBI patients from 15 randomized controlled trials (RCTs). We found that HTS was significantly more effective than other ICP-lowering agents with RR of 0.74 (95% CI: 0.58–0.94) for reduction of elevated ICP; RR = 0.57 (95% CI: 0.40–0.81) for all-cause mortality; RR = 0.68 (95% CI: 0.49–0.95) for rate of adverse hypernatremia; RR = 0.73 (95% CI: 0.60–0.88) for substantial change in the Glasgow Outcome Scale (GOS) score and shorter period of hospital stay with MD of –1.26 (95% CI: –2.30 to –0.21).
Conclusions. We found that HTS is considerably effective in reducing elevated ICP with improvement in long-term neurological functions, all-cause mortality, rate of hypernatremia, and length of hospital stay in TBI patients.
Key words: hypertonic saline, intracranial pressure, traumatic brain injury, neurological outcomes, mortality rates
Introduction
Traumatic brain injury (TBI) occurs when the brain is damaged due to a sudden, external and forceful impact1 often caused by serious sports-related accidents or vehicular collisions.2 This condition is a leading cause of fatalities and permanent disabilities in adults worldwide.3 Possible symptoms of TBI include cognitive disorientation, impaired visual acuity and difficulty concentrating, which may manifest either promptly or with a delay.4 Following a TBI, blood leakage from blood vessels between the meninges leads to the development of subdural hematomas5 and an increase in intracranial pressure (ICP),6 which in turn increases the risk of brain herniation and is associated with worse clinical outcomes. Most TBI fatalities result from an uncontrolled increase in ICP, which often occurs within the first 48 h following the event.7
Emergency care for moderate-to-severe TBI emphasizes ensuring that the patient has enough oxygen and blood flow, keeping blood pressure stable and preventing further head or neck injury.8 Therefore, reducing ICP using potential hyperosmolar treatments, including mannitol and hypertonic saline (HTS), is a critical part of treating patients with mild-to-severe TBI.9 The initial rapid infusion of large volumes of mannitol and a hypertonic crystalloid solution helps restore blood pressure and blood volume.10 A hyperosmolar solution changes the viscosity and microcirculation of blood, causes the pial arteriolar constriction, creates an osmotic gradient that pulls cerebral edema fluid from brain tissue into the bloodstream, and lets the cerebrospinal fluid leave the brain and lowers the ICP.11, 12
Since TBI severely impairs the quality of life. Researchers are investigating numerous therapeutic approaches to address this issue. Hypertonic saline is an osmotic agent that can be beneficial to patients during the acute phase of severe TBI as it reduces the detrimental consequences of secondary brain injury and regulates ICP by extracting fluid from enlarged cerebral tissue.13, 14 Nevertheless, the most recent guidelines from the Brain Trauma Foundation 201615 state that there is “insufficient evidence available from comparative studies to support a formal recommendation” for the use of HTS, even though it is becoming more popular in this setting and earlier studies have shown its clinical benefits. One possible side effect linked to HTS use is severe hypernatremia.16
Therefore, researchers have conducted numerous randomized clinical trials (RCTs), comparing infusions of different hyperosmolar treatments with HTS to investigate their efficacy in terms of reducing ICP in patients suffering from acute TBI. However, the impact of a continuous HTS infusion on neurological function, long-term functional results, all-cause mortality, long-term ICP management, and adverse effects as compared to standard treatments is still not clear. Therefore, in the present study, 15 RCTs17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 comparing the effect of HTS with other ICP-lowering hyperosmolar treatments were selected according to predefined inclusion-exclusion criteria and meta-analyzed.
Objectives
The aim of this systematic review and meta-analysis was to assess the effectiveness of HTS on the reduction of elevated ICP in patients with TBI.
Materials and methods
Search strategy and selection criteria
This meta-analysis and systematic review comply with the reporting standards established in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.32 We conducted a systematic review of RCTs that compared the efficacy of HTS with other ICP-reducing agents in the reduction of elevated ICP in patients with TBI. The investigation concentrated on individuals of all ages who were experiencing elevated ICP as a result of TBI.
The primary outcome of interest was the change in Glasgow Outcome Scale (GOS) scores after 6 months, all-cause mortality, rate of hypernatremia, rate of uncontrolled ICP, and length of hospital stay. The investigation did not include trials that involved animal studies, studies that were not randomized, or studies that did not provide hyperosmolar prophylaxis. There were no restrictions regarding language or year of publication. We conducted an exhaustive search of the scientific literature databases Embase, PubMed, Scopus, and Cochrane Library for publications released prior to April 30, 2024.
The following search terms were employed: “Hypertonic Saline” OR “HTS” OR “Intracranial Pressure Lowering Agents” OR “Traumatic Brain Injury” OR “TBI” OR “Intracranial Pressure” OR “ICP” OR “Glasgow Outcome Scale (GOS) score”, “All-cause mortality” OR “total length of stay” OR “Osmotherapy” OR “reduction in uncontrolled ICP” OR “Hypernatremia” OR “Mannitol,” OR “Mechanical ventilation” OR “Intravenous bolus infusion” OR “Cerebral perfusion pressure” OR “Randomized controlled trial” OR “RCT” OR “Systematic review” OR “meta-analysis”. Keywords were identified and evaluated for agreement in both the MEDLINE and Embase databases in accordance with the PICOS criteria.33
The keywords that were specified were inserted into the Title (ti)-Abstract (abs)-Keyword (keyword) field during the Scopus search. The Cochrane Library database employed the search keywords “traumatic brain injury,” “elevated intracranial pressure” and “hypertonic saline.” The PICO framework was employed to establish precise selection criteria. The letter “P” was employed to identify patients who had experienced TBI. Hypertonic saline was implemented by the intervention group to mitigate elevated ICP. Mannitol and other ICP-lowering agents were represented by the letter “C.” The change in GOS scores at 6 months, all-cause mortality, rate of hypernatremia, rate of uncontrolled ICP, and duration of hospital stay associated with the use of HTS compared to control agents were the primary clinical outcomes, denoted by “O.”
The design of the study was confined to the application of RCTs. The methodology utilized in our investigation was based on the approach used in the formulation of the World Health Organization (WHO) guideline.34 Further articles were discovered by employing backward and forward citation tracking on previously published meta-analyses and the studies included therein.
Table 1,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 delineates the comprehensive search strategy. The titles, abstracts and full texts of potentially qualifying publications were independently evaluated by 2 reviewers, X.R. and S.L. Any discrepancies between the 2 reviewers were resolved through discussion, and the 3rd author (J.G.) was consulted as needed.
Data analysis
The current investigation encompassed studies that provided comparative data on the efficacy of HTS compared to other ICP lowering agents in the reduction of elevated ICP in TBI patients. The studies were selected on the basis of their ability to provide full texts and an adequate amount of data for a 2×2 table. Bibliographic references that were outdated, anecdotal or wholly expert-based were excluded from the examination process. The demographic profiles of the patients and event data, including relevant components, were independently collected from the studies included in the analysis by 2 researchers (X.R. and S.L.). The data were collected using a predetermined form and comprised the following: the authors, publication year, country, total number of patients, age of patients, condition of patients at admission, intervention and control doses, and primary and secondary outcomes. In the event that the publishers’ data was insufficient or ambiguous, they were contacted to obtain supplementary information. For instance, clarification was sought when the dosage of ICP lowering agents was unclear. All-cause mortality, rate of hypernatremia, rate of uncontrolled ICP, length of hospital stay, and change in GOS scores at 6 months were the primary outcomes evaluated.
Risk of bias assessment of included studies
The researchers employed a standardized questionnaire to evaluate the studies under investigation for any potential biases. The Cochrane Risk-of-Bias tool v. 2 was employed by 2 authors to independently assess the risk of bias in individual investigations.35 Five components comprised the tool: bias induced by randomization, bias resulting from deviations from intended interventions, bias due to lacking outcome information, bias during outcome evaluation, and bias in selecting out the reported outcomes. In order to evaluate potential bias, 2 researchers (X.R. and S.L.) conducted an impartial evaluation. An additional reviewer (J.G.), assumed the role of an arbitrator to resolve any remaining disputes. Ultimately, the potential bias was evaluated and classified as either “uncertain risk”, “high risk” or “low risk”.
Statistical analyses
A comparison-adjusted funnel plot was employed to evaluate publication bias and small-study effects.36 Begg’s test37 was implemented by MedCalc software v. 23.1.7 (MedCalc, Ostend, Belgium).38 to verify the statistically significant impact of this bias. The software program Review Manager (RevMan) v. 5.4 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark)39 was implemented to assess and analyze the impact of various continuous and dichotomous outcomes. In order to evaluate binary outcomes, relative risks (RRs) were calculated for each study, along with 95% confidence intervals (95% CIs)40 and the mean difference (MD).41 The risk ratio (RR) was computed using the DerSimonian-Lair method42 and a 2×2 table43 that contained event data. The quantitative evaluation excluded studies that did not report any selected primary or secondary outcome. The objective of forest plots44 were created to assess the impact of various outcome determinants. The heterogeneity was evaluated using statistical methods, including the I2 test45 and the χ2 test,46 which were used in conjunction with a p-value. A random effect model47 was implemented due to the fact that the investigations were conducted in different settings. Statistical significance is defined as a p-value that is less than 0.05.48 In order to evaluate the efficacy of HTS in reducing elevated ICP in acute TBI patients in comparison to control agents, a subgroup analysis was conducted.
Results
Study selection outcomes
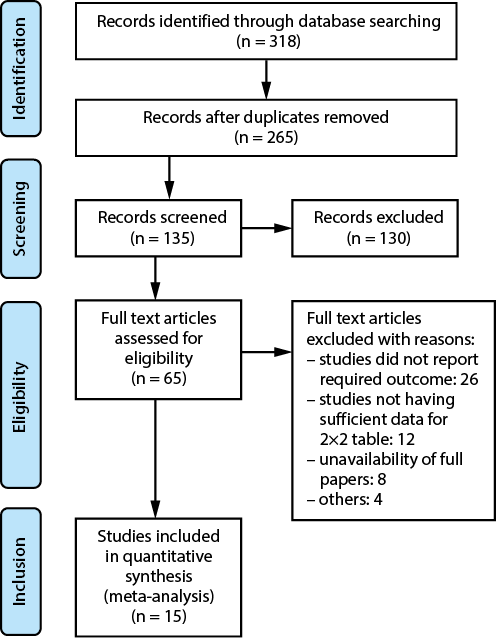
An extensive electronic survey was done by searching across multiple databases. A total of 318 papers were identified that satisfied the inclusion criteria specified in the PICOS criteria. Out of the 265 articles that were considered, 53 papers were excluded because they had duplicate content or titles and abstracts that were not relevant. After conducting additional screening, a total of 135 papers were then evaluated to determine their eligibility. However, after applying the inclusion-exclusion criteria, it was determined that 130 research did not meet the requirements and were consequently excluded. Subsequently, the remaining 65 articles were assessed to determine their suitability. Among the whole pool of studies examined, 50 were eliminated, mostly because they did not match the inclusion criteria, did not provide enough data to create 2×2 tables or did not have significant outcome measures. Ultimately, this meta-analysis included a total of 15 RCTs that met the predefined inclusion-exclusion criteria, as depicted in Figure 1. The researches included in the analysis involve a combined total of 965 participants, spanning various age groups. The salient features of the publications considered in this meta-analysis are outlined in Table 2. The text provides information on the authors’ identification and the year of publication, the study design, the condition of the patients, the total number of patients, the age of the participants, the dosage of the intervention and comparator, as well as the primary and secondary outcomes. In addition, data regarding the events in the 2×2 table were obtained from the aforementioned research for the purpose of conducting a meta-analysis.
Quality assessment of the included studies
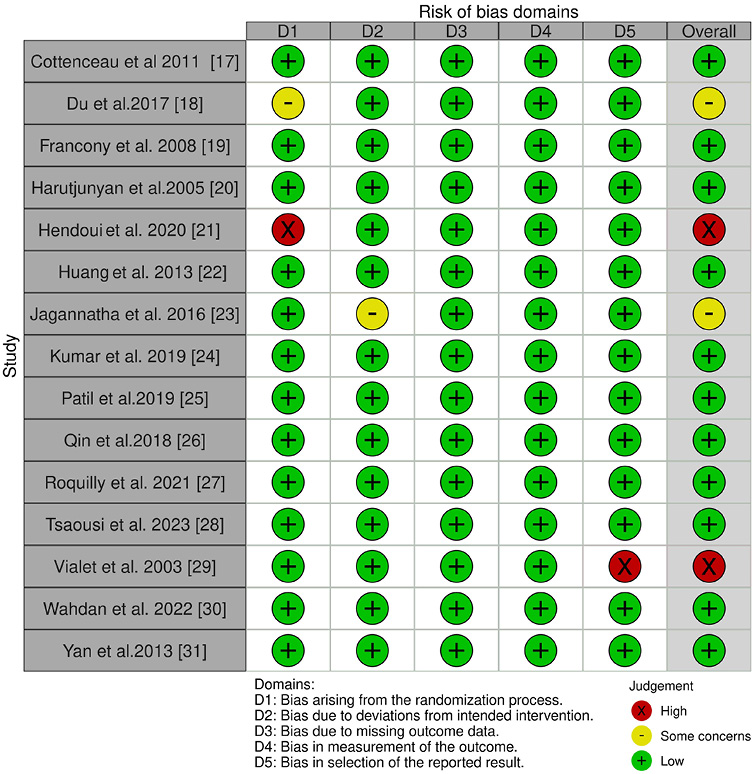
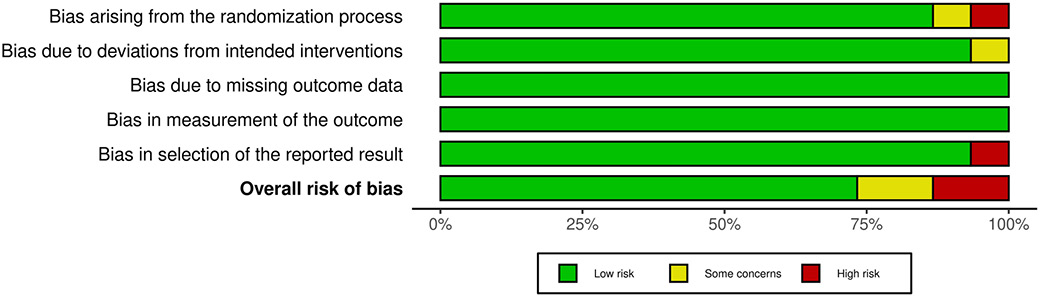
An assessment of potential risk of bias was executed out to calculate the overall rating for the study’s quality. Table 3 displays the results of the risk of bias assessment for each of the 15 RCTs that were included, using the predetermined questionnaire. The current meta-analysis exhibits a low risk of bias, as indicated by the traffic light plot in Figure 2 and the summary plot for bias assessment displayed in Figure 3. Among the 15 RCTs, 11 studies were determined to have a low risk of bias.
Two RCTs, of Du et al.18 and Jagannatha et al.,23 demonstrate a moderate level of bias. This is attributed to issues with the randomization method and the deviation from the intended intervention, respectively. The other 2 RCTs conducted by Hendoui et al.21 and Vialet et al.29 demonstrate a high risk of bias pertaining to the randomization method and the selection of reported outcomes, respectively.
Findings derived from the statistical investigation
In all, 965 TBI patients from 15 selected RCTs were included in the current meta-analysis to evaluate the efficacy of HTS compared to other ICP-lowering agents on the reduction of elevated ICP in patients with TBI. The following conclusions were obtained from the statistical analysis of the primary study outcome:
Comparison of the efficacy of HTS and other ICP lowering agents in controlling the elevated ICP in TBI patients
The included studies defined the elevated ICP as a requirement of “stage 3 therapies” in accordance with the Brain Trauma Foundation guidelines, which include barbiturates to reduce ICP.11 Elevated ICP is defined as the “persistently elevated ICP greater than 20 mm Hg despite a maximum of 3 doses of hyperosmolar therapy,” necessitating the use of further ICP-lowering measures, such as hyperventilation, propofol, cerebrospinal fluid drainage, or decompressive craniectomy.49
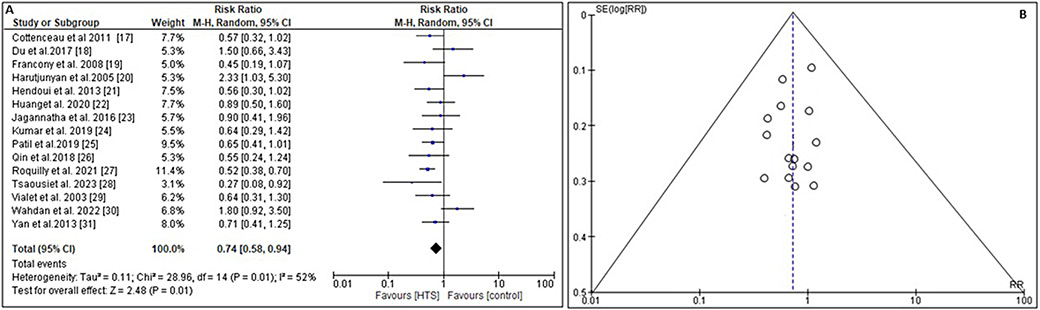
Treatment failure is defined as a sustained raised ICP greater than 35 mm Hg despite 2 consecutive infusions of hyperosmolar or an average time ICP exceeding 20 mm Hg, barbiturate requirement, and/or episodes of refractory ICP after 3 consecutive doses of hyperosmolar therapy.50 In order to evaluate the overall efficacy of HTS in managing the rate of elevated ICP in TBI patients in comparison to other ICP lowering agents, the RR and 95% CI were calculated using event data from the included studies (Figure 4). This meta-analysis demonstrated that HTS was significantly more effective than other agents in reducing ICP (RR = 0.74, 95% CI: 0.58–0.94, Tau2 = 0.11, χ2 = 28.96, degrees of freedom (df) = 14, I2 = 52%, Z = 2.48, and p = 0.01) as shown in Figure 4A. Additionally, the symmetrical funnel diagram in Figure 4B and a statistically insignificant p-statistic of Begg’s test (p = 0.312), which exceeds the predefined significance threshold of 0.05, suggested a minimal likelihood of publication bias.
Subgroup analysis
A subgroup analysis was conducted to investigate the effectiveness of HTS and other ICP lowering agents in controlling elevated ICP in TBI patients. The analysis focused on evaluating changes in GOS scores at 6 months, all-cause mortality, the rate of hypernatremia, and the length of hospital stay.
All-cause mortality
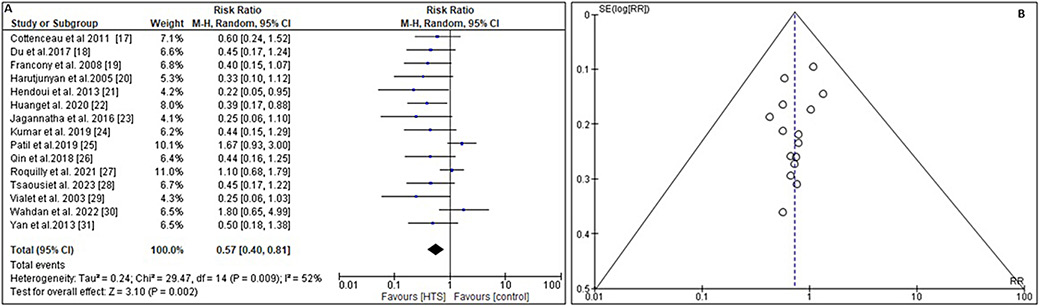
To determine the impact of infusing HTS vs other ICP lowering comparator on all-cause mortality, the RR and 95% CI were calculated using the event data extracted from the included trials (Figure 5A). In comparison to the comparator, the administration of HTS results in a lower mortality rate with an RR of 0.57 (95% CI: 0.40–0.81) and Tau2 value of 0.24, χ2 value of 29.47, df = 14, Z = 3.10, I2 = 52%, and p = 0.002. In addition, the symmetrical funnel plots (Figure 5B) and the statistically insignificant p value (p = 0.104) from Begg’s test, (>0.05) indicate a low probability of publication bias.
Rate of adverse hypernatremia
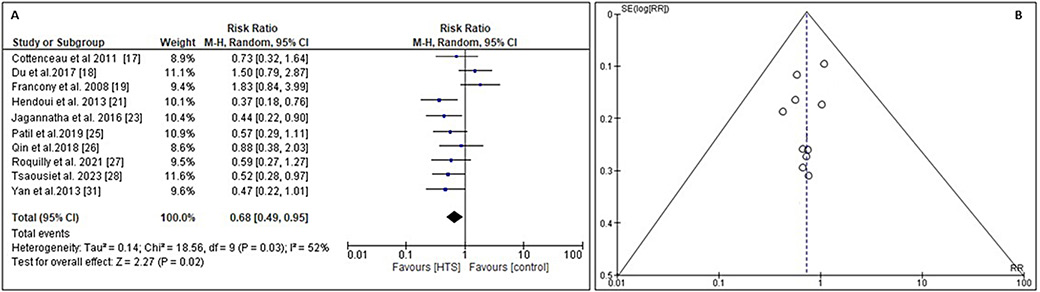
To find out the impact of infusing HTS vs other ICP lowering comparator on the rate of adverse hypernatremia or defined as a rise in serum sodium concentration to a value exceeding 145 mmol/L,51 the RR and 95% CI were calculated using the event data extracted from the included RCTs (Figure 6A). In comparison to the comparator, the administration of HTS results in lower rate of adverse hypernatremia with an RR of 0.68 (95% CI: 0.49–0.95) and Tau2 value of 0.14, χ2 value of 18.56, df = 9, Z = 2.27, I2 = 52%, and p = 0.02. Moreover, the symmetrical funnel plots depicted in Figure 6B, along with the statistically negligible p-value (p = 0.284) obtained from Begg’s test (p > 0.05), suggest a little likelihood of publication bias.
Change in GOS scale score
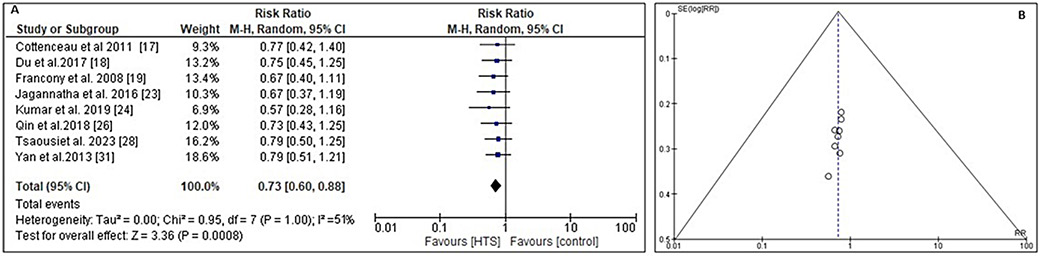
The GOS score52 is an ordinal scale used to assess patients’ functional outcomes following brain injury, taking into account the patients’ level of consciousness and ability to perform activities of daily living (ADLs). The RR and 95% CI were calculated using the event data extracted from the included RCTs to determine the impact of infusing HTS vs other ICP lowering comparator on the change in the GOS score (Figure 7A). Compared to the comparator, the administration of HTS leads to a substantial change in GOS scale score. The relative risk (RR) is 0.73 (95% CI: 0.60–0.88), with a Tau2 value of 0.00, χ2 value of 0.95, df = 7, Z-score of 3.36, I2 value of 51%, and a p < 0.001. In addition, the symmetrical funnel plots shown in Figure 7B, coupled with the statistically insignificant p-value of 0.144 obtained from Begg’s test (p > 0.05), indicate a low probability of publication bias.
Length of hospital stay
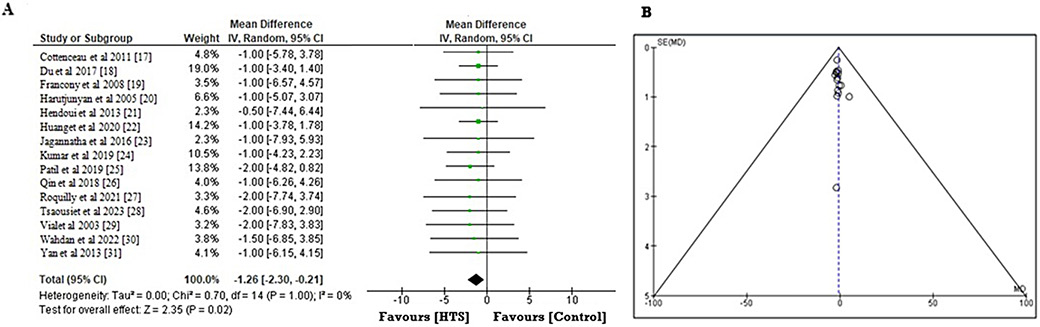
To investigate the beneficial impact of infusing HTS compared to other ICP lowering comparator on the total length of hospital stay, the MD and the 95% CI were calculated using the event data extracted from the included RCTs (Figure 8A). Compared to the comparator, the administration of HTS leads to a shorter period of hospital stay. The estimated MD is –1.26 (95% CI: –2.30 to –0.21), with a Tau2 value of 0.00, χ2 value of 0.70, df = 14, Z = 2.35, I2 = 70%, and p = 0.02. In addition, the symmetrical funnel plots shown in Figure 8B, combined with the statistically insignificant p-value of 0.246 calculated from the Begg’s test (p > 0.05), stipulate a low probability of publication bias.
From the abovementioned statistical analysis of the included RCTs,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 we found that HTS was significantly more effective than other hyperosmolar agents in reducing ICP (RR = 0.74 (95% CI: 0.58–0.94)), mortality rate (RR = 0.57 (95% CI: 0.40–0.81)) and rate of adverse hypernatremia (RR = 0.68 (95% CI: 0.49–0.95)), providing substantial change in GOS scale score (RR = 0.73 (95% CI: 0.60–0.88)), with shorter period of hospital stay (MD = –1.26 (95% CI: –2.30 to –0.21)). These values of RR 1 indicate that HTS is substantially more effective than other ICP-lowering agents for improving long-term neurological function, all-cause mortality and length of hospital stay in patients with acute TBI.
Discussion
According to our systematic review and meta-analysis including 15 RCTs17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 that incorporated 965 TBI patients of all age groups, HTS was found to be considerably more effective than other ICP-lowering agents. The following are the primary findings that were determined: 1) HTS was substantially more effective than primarily mannitol in improving the long-term neurological outcome in patients with elevated ICP; 2) HTS was more effective in lowering the all-cause mortality and length of hospital stay and reducing risk of adverse hypernatremia. The wide 95% CIs for all the evaluated outcomes indicate the therapeutically significant differences between HTS and other hyperosmolar treatments for decreasing ICP, such as mannitol. Our findings are in accordance with the findings of a systematic review and meta-analysis conducted by Bernhardt et al.,53 who concluded that there is no evidence of an effect of HTS on clinically significant outcomes and that HTS is associated with adverse hypernatremia. In spite of the fact that HTS was found to be not related to deleterious hypernatremia when compared with other agents, this result must be viewed with care due to the fact that the majority of the weighting for this point estimate is based on a single large multicenter trial.27 This research was significant since it explored the continuous infusion of a larger dosage of HTS (20%) than is often used in clinical settings (range: 1.8–5%). The infusion was administered for a minimum of 48 h. Therefore, it is conceivable that the apparent increased risk of hypernatremia in the patients who were investigated is mostly because of the lengthy continuous infusion of concentrated HTS. Similarly, our findings are align with recent practice surveys, which revealed that most centers use HTS as first-line hyperosmolar therapy rather than mannitol.54, 55
However, our findings contradict the findings of prior research that suggested that HTS is not more successful than its comparators (e.g., mannitol), such as Chen et al.,56 who conducted a Cochrane review in which they analyzed trials that compared HTS to a potential range of other ICP-lowering agents, including mannitol or mannitol in combination with glycerol. They contended that HTS is not superior to mannitol in terms of efficacy and safety in the long-term management of acute TBI, based on the limited data and weak evidence. The majority of their included RCTs were at a high or ambiguous risk of bias due to selective reporting, incomplete outcome data and a lack of blinding. Additionally, certain studies have indicated that there is no difference in plasma sodium concentration between patients who are getting HTS and those who are receiving mannitol boluses. This difference may indicate that certain delivery procedures are not successful in achieving a hyperosmolar state.57, 58 The effect of bolus compared to continuous infusion of HTS on plasma sodium levels should be investigated further to discover whether or not there is an optimal administration route that can generate a therapeutic hyperosmolar state without causing deleterious hypernatremia. Continued research is required to answer this question. It is likely that using near-patient salt monitoring, such as blood gas analysis, will make it easier for clinicians to give and alter the dosage of HTS.
Within the setting of TBI, there is a dearth of large-scale RCTs that compare ICP-lowering treatments due to the relatively low occurrence of severe TBI in critical care settings, which necessitates the use of ICP-lowering medicines. Similarly, RCT data from nations with lower middle incomes and RCT involving pediatric populations are scarce. In this particular review, for example, there is only 1 pediatric trial that reports a GOS score of ≤12 and primarily included trials involving adult patients from higher-income countries.21 In order to address the existing lack of high-quality evidence and to ascertain whether or not HTS is a preferred ICP-lowering agent in particular patient population, there is a pressing need for larger international and multicenter trials to be conducted in a range of contexts.
Finally, the inconsistent reporting of outcomes following TBI among clinical trials, such as long-term functional outcome ratings using the GOS score, undermines the validity of comparisons between studies and impedes advancement in this area of research. For instance, 3 studies reported GOS scores in formats that were not suitable for inclusion in a pooled analysis. Vialet et al.29 only provided statistics on the number of patients who had died or had severe disability at the 90-day point. In the RCT by Jagannatha et al.,23 the term “favorable” outcome was defined as “good recovery,” “moderate disability” or “severe disability.” This definition is likely to be in conflict with what the majority of patients would view as a favorable outcome. Similarly, Kumar et al.21 recorded the number of patients who survived with or without disability, as well as the number of patients who were in a vegetative state or had passed away during the first 6 months of their illness. It is not certain whether these methods of GOS reporting will be beneficial to clinicians or patients, which highlights the importance of having a core outcome set that is standardized for TBI.
Therefore, there is an obvious need for the execution of more trials in substantial TBI (COSTS-TBI) projects59, 60 in the future for establishing a standard set for analyzing patients with moderate to severe TBI and to provide more accurate evaluations of blood pressure-lowering medicines in a variety of critical care settings, the thresholds for hazardous hypernatremia, the optimal range of plasma sodium concentration, and other clinical characteristics of patients.
Limitations
The limitations of this analysis can be attributed to the clinical and methodological disparities among trials, which also encompassed predominantly small sample sizes. In addition, variations in the methodologies used to report outcomes restricted the available data that could be included in meta-analyses. Furthermore, the subgroup analyses were based on different age groups, degrees of TBI and methods of administration. Additionally, it remains uncertain whether there is an optimal hyperosmolar therapy that varies according to the age group of the patient or the severity of TBI. Along with this, the classification between “favorable” and “unfavorable” outcomes was based on what most patients and physicians would agree upon. Therefore, it is conceivable that significant data regarding the long-term neurological prognosis, which could potentially impact and direct decisions made by patients and clinicians, may not be reflected in these findings.
Conclusions
This systematic review and meta-analysis provide substantial evidence that HTS is more effective to other hyperosmolar agents, such as mannitol, in decreasing ICP, enhancing neurological outcomes (measured using GOS score), reducing overall mortality, and shortening the duration of hospital or ICU stay in patients with acute TBI. However, this conclusion is derived from a limited number of trials with a small sample size, necessitating a comprehensive investigation into the potential benefits of HTS and the risks associated with hypernatremia. Hence, it is imperative to carry out future research that includes a substantial number of trials and a sufficient sample size in order to ensure the accuracy and reliability of the findings regarding the effectiveness of HTS for acute TBI.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.