Abstract
Background. Even though ongoing intervention is essential, several uncertainties remain about the management of intraoperative pressure wound ulcers in breast cancer patients.
Objectives. To evaluate the impact of the ongoing intervention for intraoperative pressure wound ulcer problems related with female breast cancer patients, a meta-analysis study was conducted.
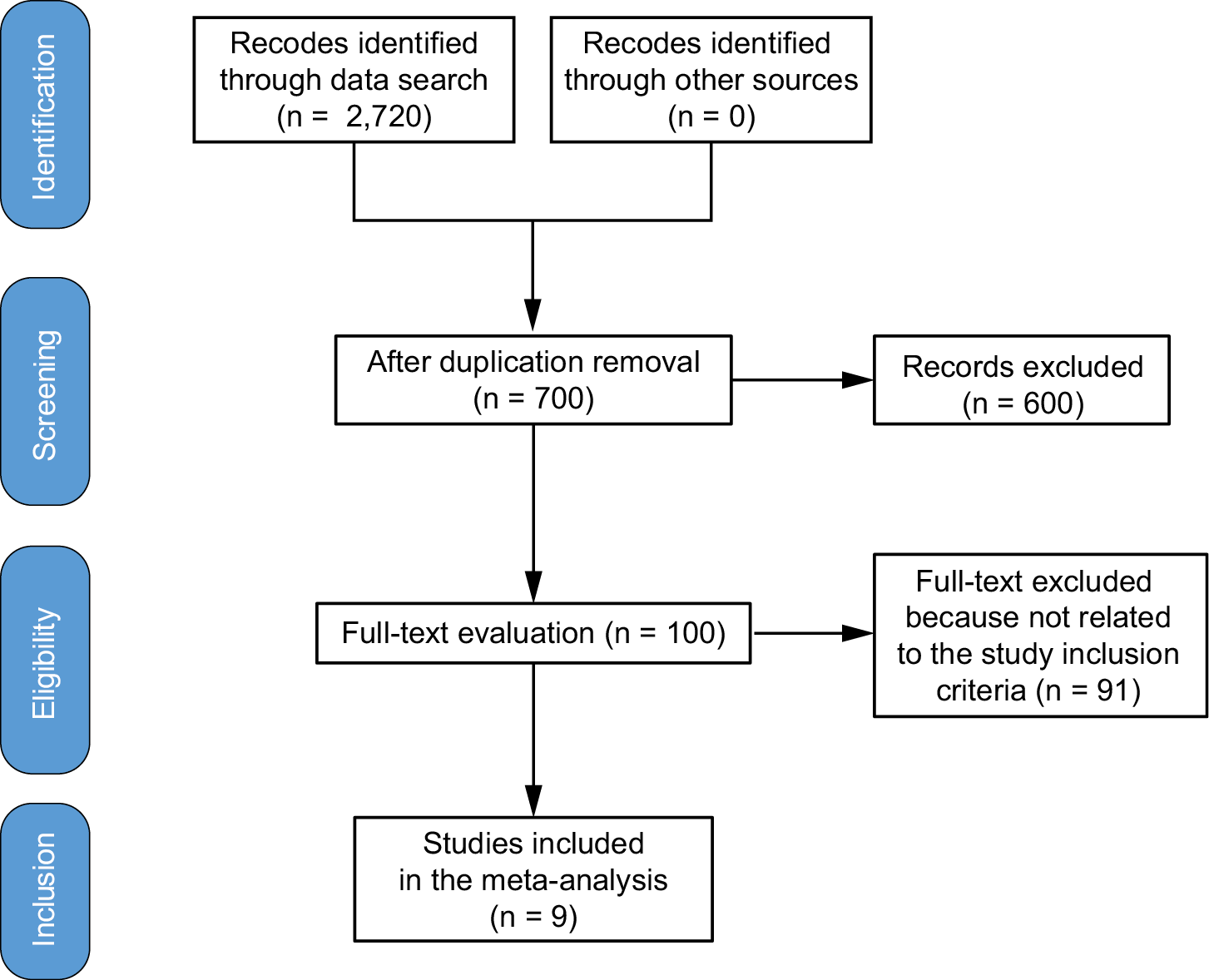
Materials and methods. Up until June 2024, comprehensive literature study was completed and 2,720 related studies were found. At the beginning point, 9 studies that were chosen included 1,467 women with breast cancer. Using dichotomous or continuous techniques and a random model, the odds ratio (OR) and mean difference (MD) and 95% confidence intervals (95% CIs) were used to evaluate the impact of continuous intervention for intraoperative pressure wound ulcers-associated difficulties in women with breast cancer.
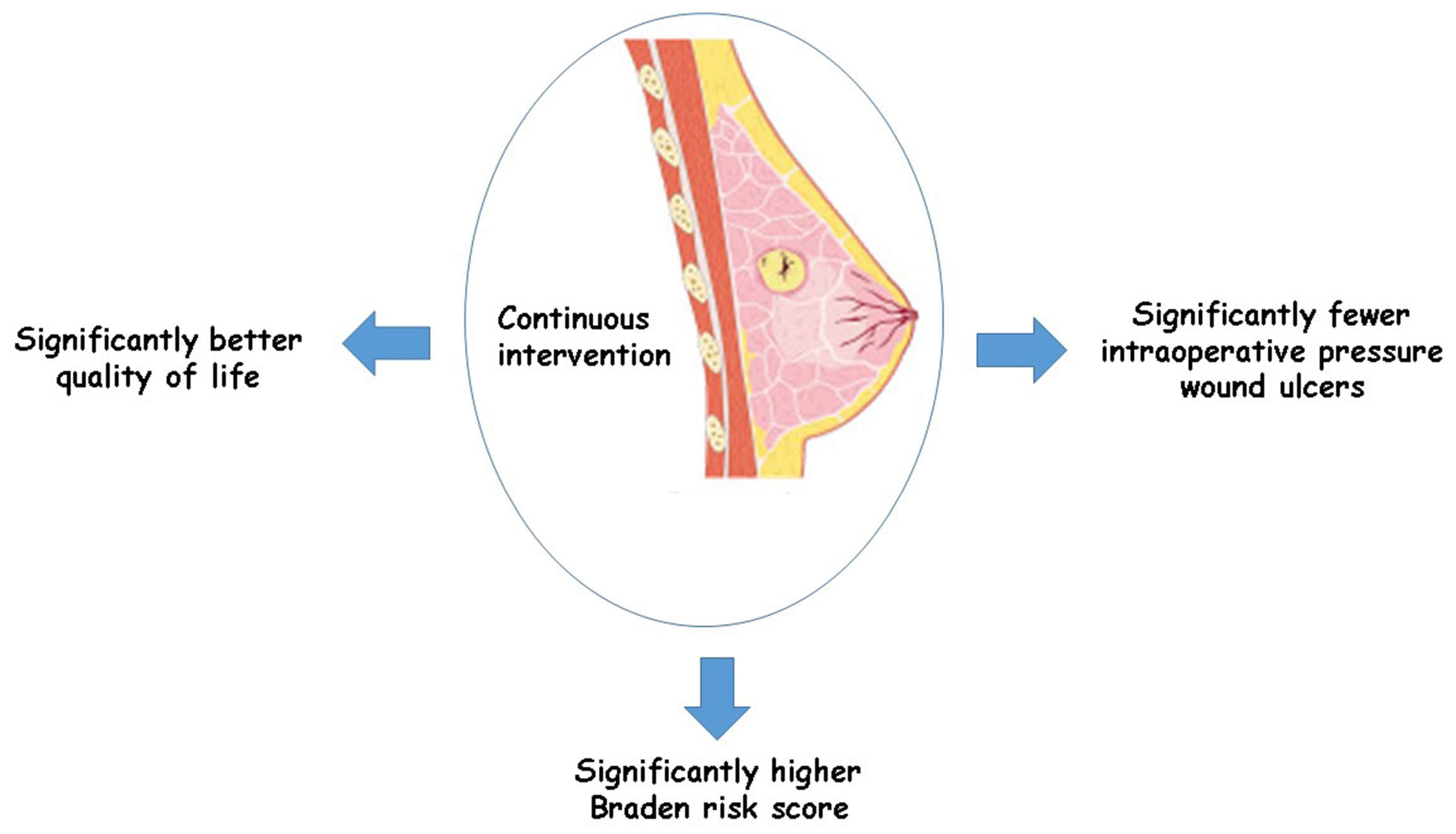
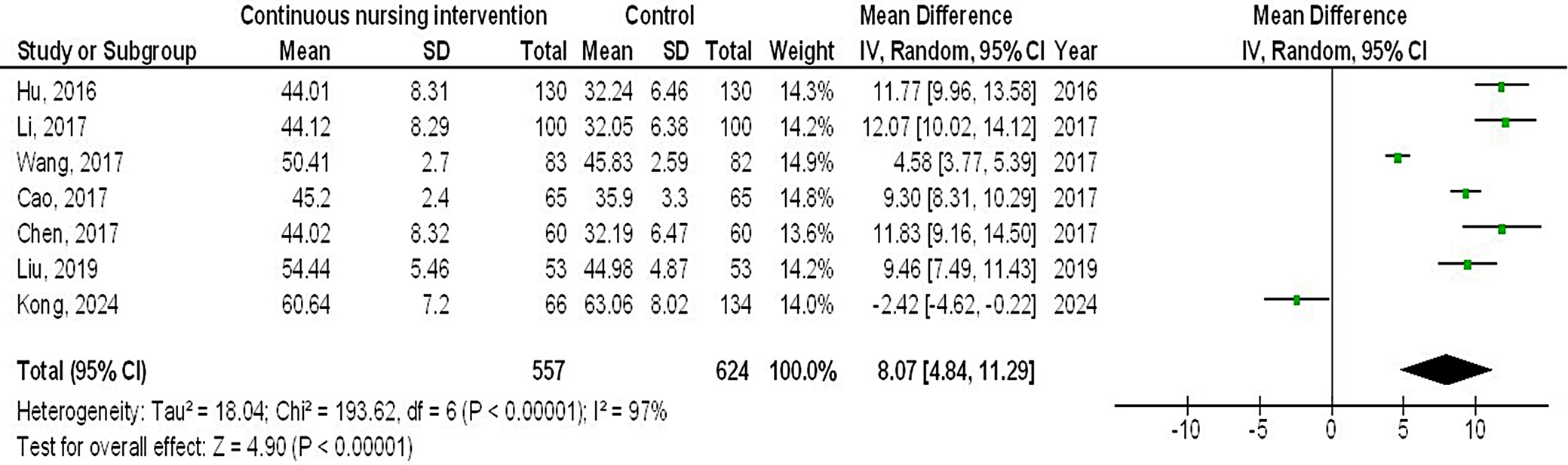
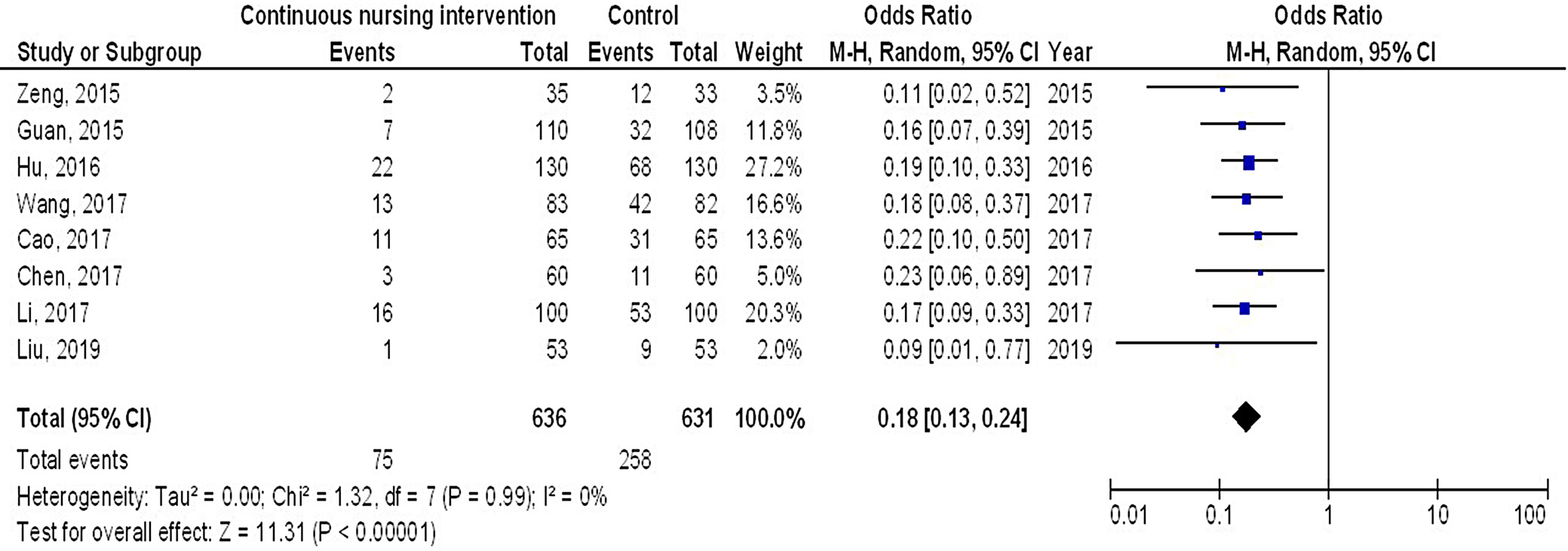
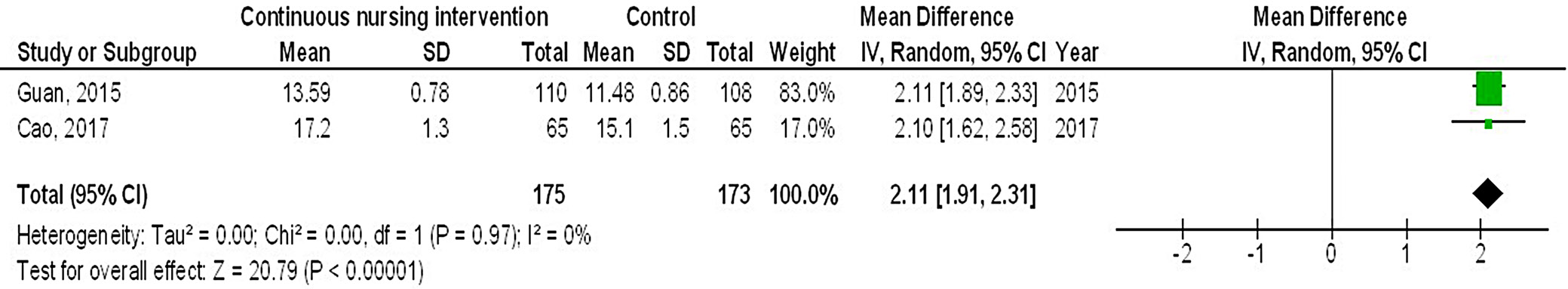
Results. In comparison to the control group of female breast cancer patients, continuous intervention resulted in significantly better quality of life (QoL) (MD = 8.07; 95% CI: 4.84–11.29, p < 0.001), fewer intraoperative pressure wound ulcers (OR = 0.18; 95% CI: 0.13–0.24, p < 0.001) and higher Braden risk score (OR = 2.11; 95% CI: 1.91–2.31, p < 0.001).
Conclusions. In comparison to the control group, women with breast cancer undergoing continuous intervention experienced a significantly better QoL fewer intraoperative pressure wound ulcers, and had a higher Braden risk score. However, because there were not many studies chosen for comparison in the meta-analysis, reader’s discretion is advised regarding its results.
Key words: breast cancer, continuous intervention, Braden risk score, intraoperative pressure wound ulcer
Background
In China, approx. 500,000 new tumor cases and 300,000 cancer-related deaths were recorded in 2024, accounting for around 30% of global tumor incidence and mortality rates, respectively.1 Malignant tumors are extremely common and have a high death rate, making them a serious threat to human health and life. In 2018, about 2 million new cases and 1.5 million deaths globally made breast cancer the most prevalent malignant tumor.2 The symptoms experienced by patients with breast cancer are intricate and varied.3 In therapeutic practice, pressure ulcers are commonly shown as a shared clinical outcome.4 Pressure wound ulcers affect millions people in the USA, the Netherlands, Germany, and Australia.5 In China, the prevalence of pressure ulcers among the population ranges between 1.14% and 1.78%.6 In addition to experiencing increased pain, patients with pressure ulcers may also suffer from feelings of depression, anxiety and loneliness. Longer hospital stays result in increased financial hardship on society and families as a result of rising hospitalization costs and social resource waste. As a result, this impacts the diagnosis and progression of the primary illness, potentially complicating treatment outcomes. Pressure wound ulcers are among the most costly medical conditions due to the high expenses associated with their management and treatment. Continuous intervention, based on a specialized technical framework, encompasses a range of activities guided by diagnosis and targeted intervention strategies. These interventions are chosen based on diagnostic characteristics, research findings, the potential for functional recovery in women, and the capabilities of both patients and healthcare providers. Young in a single-center randomized controlled trial (RCT) demonstrated that the experimental group had a significantly lower incidence of pressure wound ulcer complications compared to the control group as a result of continuous care.7 Nevertheless, this study showed that pressure ulcer complications in breast cancer patients after surgery were inevitable, depending on the length of bed rest and whether continuous care was provided.8
Caregivers must take appropriate preventive measures to reduce the risk of pressure wound ulcers and enhance the quality of life (QoL) for immobile patients. There is a positive correlation between the behavior of primary caregivers and the severity of pressure ulcers.9 Pressure wound ulcers increase the strain on patients and caregivers. It is critical to consider and investigate strategies that improve patients’ QoL while reducing the incidence of pressure wound ulcers in those with advanced breast cancer. The usefulness of continuous care for breast cancer patients who are having issues related to intraoperative pressure wound ulcers is a hotly debated topic. Therefore, to evaluate and resolve this issue, a comprehensive meta-analysis is necessary. Even though ongoing intervention is essential, several uncertainties remain about the management of intraoperative pressure wound ulcers in breast cancer patients.
Objectives
We investigated the efficacy of continuous management in preventing intraoperative pressure wound ulcers in women with breast cancer using the meta-analysis approach.
Methods
Eligibility criteria
To provide an overview, studies demonstrating how continuous intervention can mitigate issues related to intraoperative pressure wound ulcers in women with breast cancer were selected.10
Information sources
Figure 1 provides a comprehensive overview of the investigation. The literature was included in the study when the following inclusion criteria were met11:
1. The study was an RCT, observational, prospective, or retrospective study.
2. The individuals under investigation were women who had breast cancer.
3. The intervention was carried out continuously.
4. The study evaluated the impact of ongoing intervention for issues related to intraoperative pressure wound ulcers in women with breast cancer.
Research on intraoperative pressure wound ulcers in women without continuous intervention, research on the characteristics of the effect of continuous intervention for problems associated with intraoperative pressure ulcers in breast cancer patients, and research that did not emphasize the significance of the comparison were all excluded.12
Search strategy
Based on the PICOS approach, a search protocol operation was identified. We classified it as follows: QoL, intraoperative pressure wound ulcers and Braden risk score were the “outcomes”, continuous intervention was the “intervention” or “exposure,” while the “comparison” was between continuous intervention and control. Finally, “research design” meant that the planned research had no boundaries.13
Until June 2024, we conducted a comprehensive search across the following databases: Google Scholar, Embase, Chinese Biomedical Literature Database, Cochrane Library, PubMed, and OVID. We did this by organizing keywords and adding more keywords related to breast cancer, intraoperative pressure wound ulcers, Braden risk score, and continuous intervention (Table 1).14, 15, 16 To ensure that the investigation accurately established a link between the impact of continuous intervention on intraoperative pressure wound ulcers in female breast cancer patients, duplicate papers were removed and compiled into an EndNote file, and their titles and abstracts were reassessed.17, 18
Selection process
The meta-analysis method was used to organize and assess the procedure that followed the epidemiological proclamation.
Data collection process
Some of the criteria used to collect data included the first author’s name, research data, year of study, country or region, population type, medical and treatment characteristics, classification categories, quantitative and qualitative evaluation methods, data sources, outcome assessments, and statistical analysis.19
Data items
Main consequences of the inclusion parameter were analyzed. All studies were conducted on females; language of publication was neither an inclusion nor an exclusion criterion. There were no restrictions on the number of volunteers who could be found for the research. As letters, reviews and editorials are not appropriate for meta-analysis, these were not included in our study.20, 21
Study risk of bias assessment
Two authors evaluated the selected papers’ methods independently to assess the possibility of bias in each study. Procedural quality was assessed using the “risk of bias instrument” from the Cochrane Handbook for Systematic Reviews of Interventions, v. 5.1.0.22.22 After each study was classified using the assessment criteria, they were categorized as having a medium bias risk if 1 or more quality requirements were not met, and as having a low bias risk if all requirements were met. The research was deemed to have a significant bias risk if multiple quality standards were either fully or partially satisfied.
Effect estimates
Sensitivity analysis was limited to studies that assessed and detailed the impact of continuous management for intraoperative pressure wound ulcer issues related with female breast cancer patients. The limited availability of demographic data, such as age and ethnicity, for comparison outcomes hindered the application of stratified models to examine the effects of specific factors.23, 24, 25
Statistical analyses
Using either dichotomous or continuous methods within a random-effects model, the odds ratio (OR) and mean difference (MD), along with their 95% confidence intervals (95% CIs), were calculated. The I² index was calculated using a range from 0% to 100%, where values of 0%, 25%, 50%, and 75% indicated no, low, moderate, and high heterogeneity, respectively.26 The analysis used a p-value of less than 0.05 to define the statistical significance of differences among subgroups.27
Reporting bias assessment
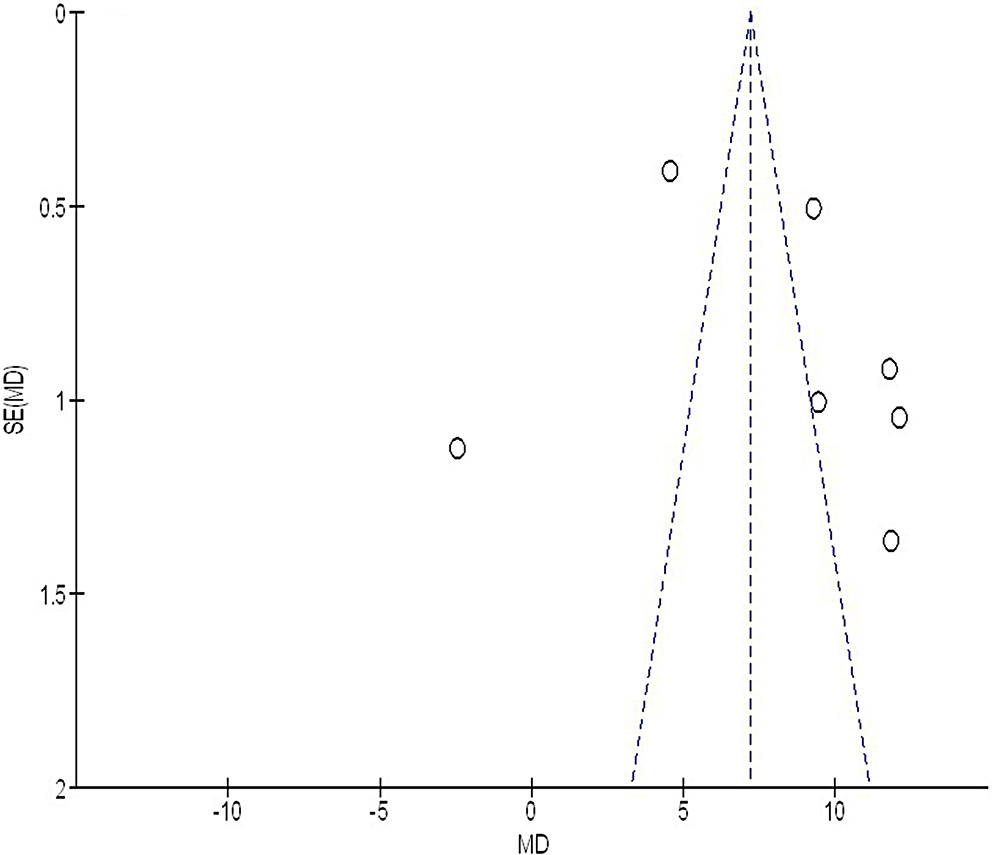
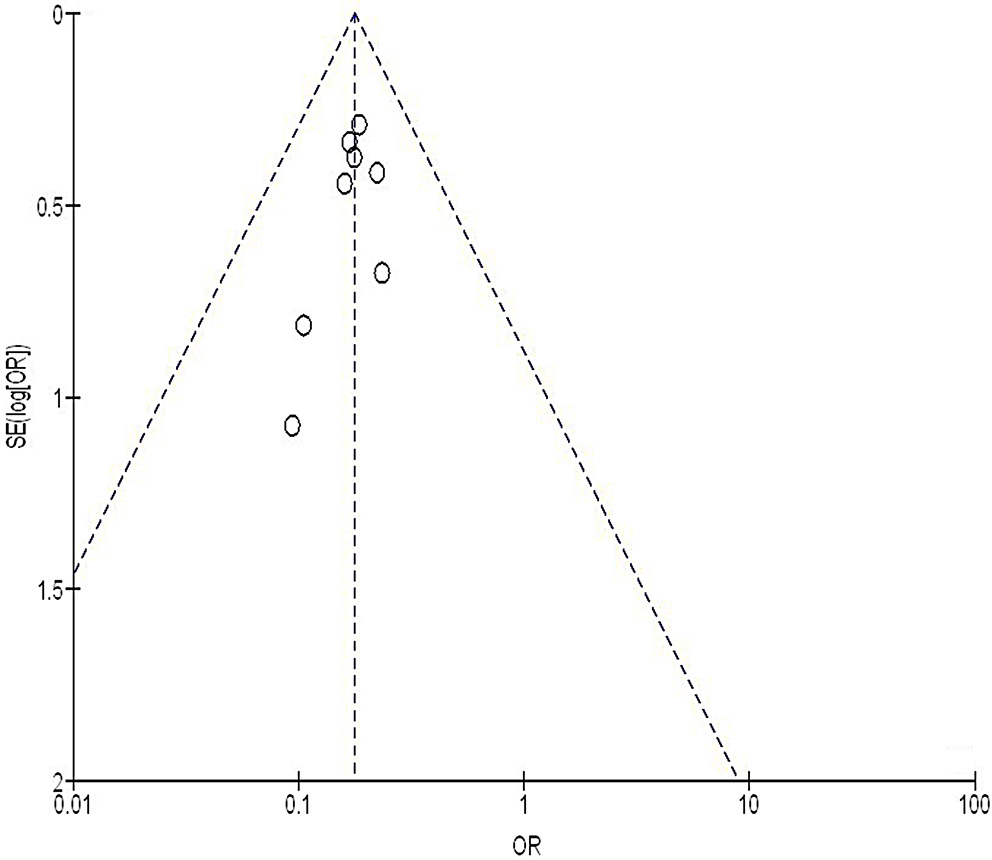
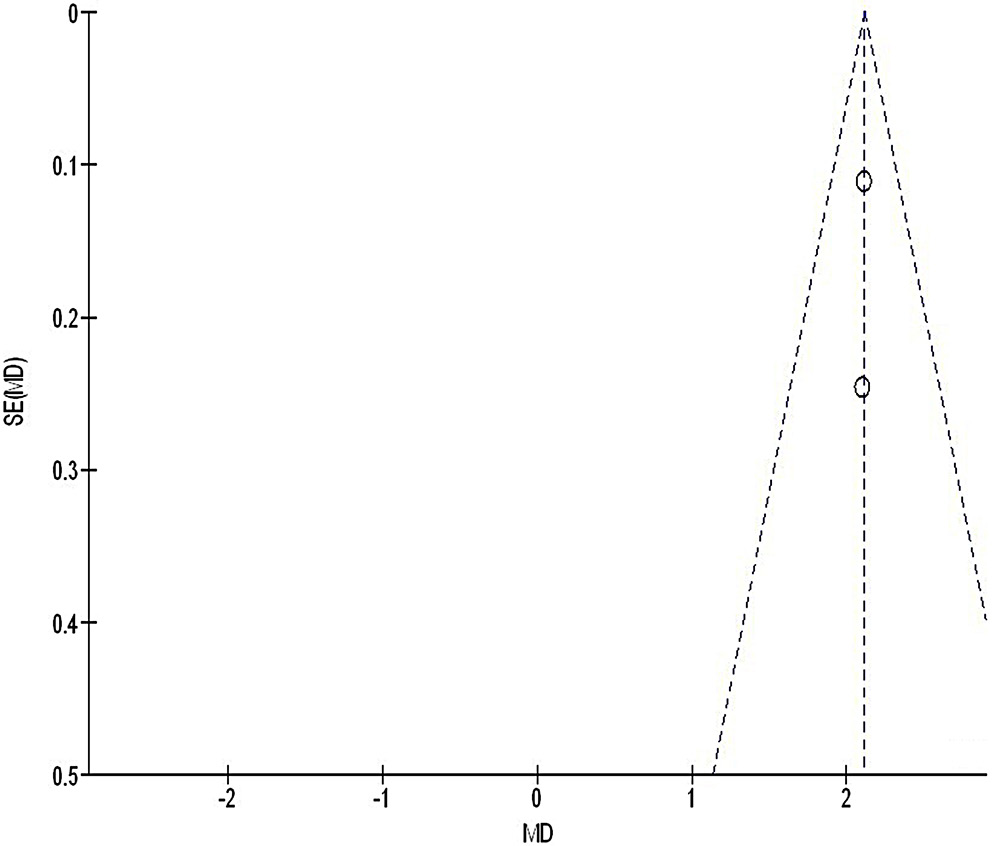
We used the Egger’s regression test and funnel plots, displaying the logarithm of the ORs against their standard errors (SEs), to quantitatively and qualitatively assess publication bias. A p-value of less than 0.05 indicated the presence of significant bias.28
Certainty assessment
Two-tailed testing was utilized to examine every p-value. Graphs and statistical analyses were produced using Reviewer Manager v. 5.3 (The Nordic Cochrane Centre, the Cochrane Collaboration, Copenhagen, Denmark).29, 30
Results
Out of 2,720 relevant studies meeting the inclusion criteria, 9 articles published between 2015 and 2024 were selected for inclusion in this analysis.31, 32, 33, 34, 35, 36, 37, 38, 39 Table 2 summarizes the findings of these studies. At the outset, the research included 1,467 women with breast cancer, of whom 702 received continuous intervention and 765 were assigned to the control group. The sample sizes across studies ranged from 68 to 260 women. Figure 2, Figure 3, Figure 4 illustrate that, compared to the control group, continuous intervention in women with breast cancer resulted in significantly improved QoL (MD = 8.07; 95% CI: 4.84–11.29, p < 0.001) with high heterogeneity (I² = 97%), a significantly lower incidence of intraoperative pressure wound ulcers (OR = 0.18; 95% CI: 0.13–0.24, p < 0.001) with no heterogeneity (I² = 0%), and a higher Braden risk score (OR = 2.11; 95% CI: 1.91–2.31, p < 0.001) with no heterogeneity (I² = 0%). Using the quantitative Egger’s regression test and visual interpretation of the funnel plots presented in Figure 5, Figure 6, Figure 7, no evidence of publication bias was detected (p = 0.90). However, as illustrated in Figure 8, while there was no bias in selective reporting, the majority of the included RCTs exhibited poor procedural quality.
Discussion
A total of 1,467 female breast cancer patients were included at the outset of the studies selected for the meta-analysis. Among them, 702 patients received continuous intervention, while 765 were in the control group.31, 32, 33, 34, 35, 36, 37, 38, 39 In comparison to the control group, female breast cancer patients undergoing continuous intervention experienced a significantly better QoL and fewer intraoperative pressure wound ulcers, and had a higher Braden risk score. However, due to the limited number of studies included in the meta-analysis for comparison, such as those utilizing the Braden risk score, caution must be exercised when interpreting the results, as this may affect the significance of the evaluated assessments.40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50
The overall death rate for breast cancer is the highest among all malignant tumors in women.51 Because of the complex and varied symptoms that affect this population, pressure wound ulcers are significantly more common in women with breast cancer. Many anti-tumor medications can lead to hypoalbuminemia, acute malnutrition, increased cancer-related pain, and other adverse effects in patients.40, 41, 44, 45 These treatments also impact patients’ nutritional metabolism, food intake, nutrient absorption barriers, tumor cell catabolism, and the biological activity of tumors. Inadequate prompt intervention might lead to the rapid formation of pressure wound ulcers.52 The community healthcare system in China is still developing and has yet to reach an optimal level. Patients with advanced breast cancer are primarily cared for at home by their relatives, and the patient’s QoL is directly affected by their understanding of pressure wound ulcers. The incidence of pressure ulcers is also strongly linked to life satisfaction.53 As patients transition from the hospital back to their families or communities, aftercare includes hospital discharge planning, referrals, ongoing follow-up, and counseling. The integration of the telemedicine-specific model and telemedicine platform, which includes the use of web-based education programs, encouraging self-management patient applications, and the peer-based patient-driven platform of pressure wound ulcer continuity care model, are the main components of the care model continuity, as reported in various experiments.54, 55 Researchers from many places are progressively employing a multidisciplinary approach to pressure wound ulcer therapy.42, 46, 47, 48, 49, 50 The guidelines for treating pressure wound ulcers include laser therapy as the lowest recommended grade of treatment. However, they also contribute to a broader understanding of the management of the disease.56 Patients’ firsthand experience with the coordination and continuation of medical treatments is what continuity of care is all about. They will be naturally encouraged by this, and it will help to promote their health.
Limitations
Assortment bias may have occurred. We also lacked the information needed to conclude whether certain characteristics, such as race and age, had an impact on outcomes. Bias may have been amplified due to the inclusion of incomplete or inaccurate data from previous studies. Factors such as age, race and nutritional status of the women were likely sources of bias. Additionally, incomplete data and unpublished research could have unintentionally led to skewed values.
Conclusions
In comparison to the control group, women with breast cancer undergoing continuous intervention experienced a significantly better QoL and fewer intraoperative pressure wound ulcers, and had a higher Braden risk score. However, due to the limited number of studies included in the meta-analysis for comparison, such as those utilizing the Braden risk score, caution must be exercised when interpreting the results. This limitation may affect the significance and reliability of the evaluated assessments.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.

















.png)