Abstract
Sleep disorders have emerged as a significant public health issue, adversely affecting quality of life and precipitating severe complications. The association between obstructive sleep apnea syndrome (OSAS) and otolaryngological manifestations appears to be underrecognized. This study posits that manifestations in the ear, nose and throat (ENT) among patients with OSAS and users of continuous positive airway pressure (CPAP) therapy are relatively common. Utilizing the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement, this systematic review, registered at PROSPERO (No. CRD42023452473), involved a comprehensive search of the MEDLINE databases. We included studies published in English from 1979 to March 2021 that explored the linkages between OSAS, CPAP and otolaryngological manifestations. A total of 29 articles were reviewed, with findings indicating 12 studies on ear dysfunctions, 11 on nose dysfunctions and 6 on pharynx dysfunctions. Reported symptoms included hearing dysfunction, vestibular function disorders, cerebrospinal fluid leak, Eustachian tube (ET) dysfunction, rhinosinusitis, olfaction and taste disorders, dysphagia, dry mouth, and gastroesophageal reflux. The etiology of these ailments varies, yet an understanding of these symptoms can improve the diagnosis to confirm or rule out OSAS. Early identification of ENT symptoms related to OSAS may facilitate prompt diagnosis and mitigate serious complications.
Key words: continuous positive airway pressure, obstructive sleep apnea, hearing dysfunction, cerebrospinal fluid leak, Eustachian tube dysfunction
Introduction
Sleep disorders have become a significant public health concern, leading to a reduction in quality of life and serious complications such as stroke, myocardial infarction, heart failure, and overall diminished life quality. Obstructive sleep apnea syndrome (OSAS) is the most prevalent sleep-related breathing disorder, affecting an estimated 936 million adults aged 30–69 globally with mild-to-severe OSAS, and 425 million adults aged 30–69 with moderate-to-severe OSAS.1 Characterized by recurrent episodes of partial or complete upper airway collapse during sleep, OSAS is typically treated with continuous positive airway pressure (CPAP), the “gold standard” for sleep apnea treatment.
Although OSAS predominantly impacts the cardiovascular and cerebrovascular systems, it can also manifest a range of otolaryngological symptoms. These issues are often overshadowed by more life-threatening problems, thus receiving less attention from both patients and healthcare providers. The association between OSAS, CPAP therapy and otolaryngological manifestations is frequently overlooked during initial examinations. Therefore, identifying ear, nose and throat (ENT) symptoms in patients with undiagnosed OSAS is crucial. This study aims to provide a systematic review of current knowledge on OSAS-related issues in the head and neck region and to assess symptoms during CPAP use.
Objectives
The objectives of this study are:
1. To systematically review and summarize the current knowledge on otolaryngological manifestations associated with OSAS.
2. To assess the prevalence and types of ENT symptoms in patients diagnosed with OSAS.
3. To evaluate the impact of CPAP therapy on the otolaryngological symptoms in OSAS patients.
4. To identify gaps in existing research and suggest areas for future studies on the relationship between OSAS, CPAP therapy and ENT symptoms.
Methods
This review was based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews2 and was registered in PROSPERO (The International Prospective Register of Systematic Reviews) under No. CRD42023452473.
Search strategy and data sources
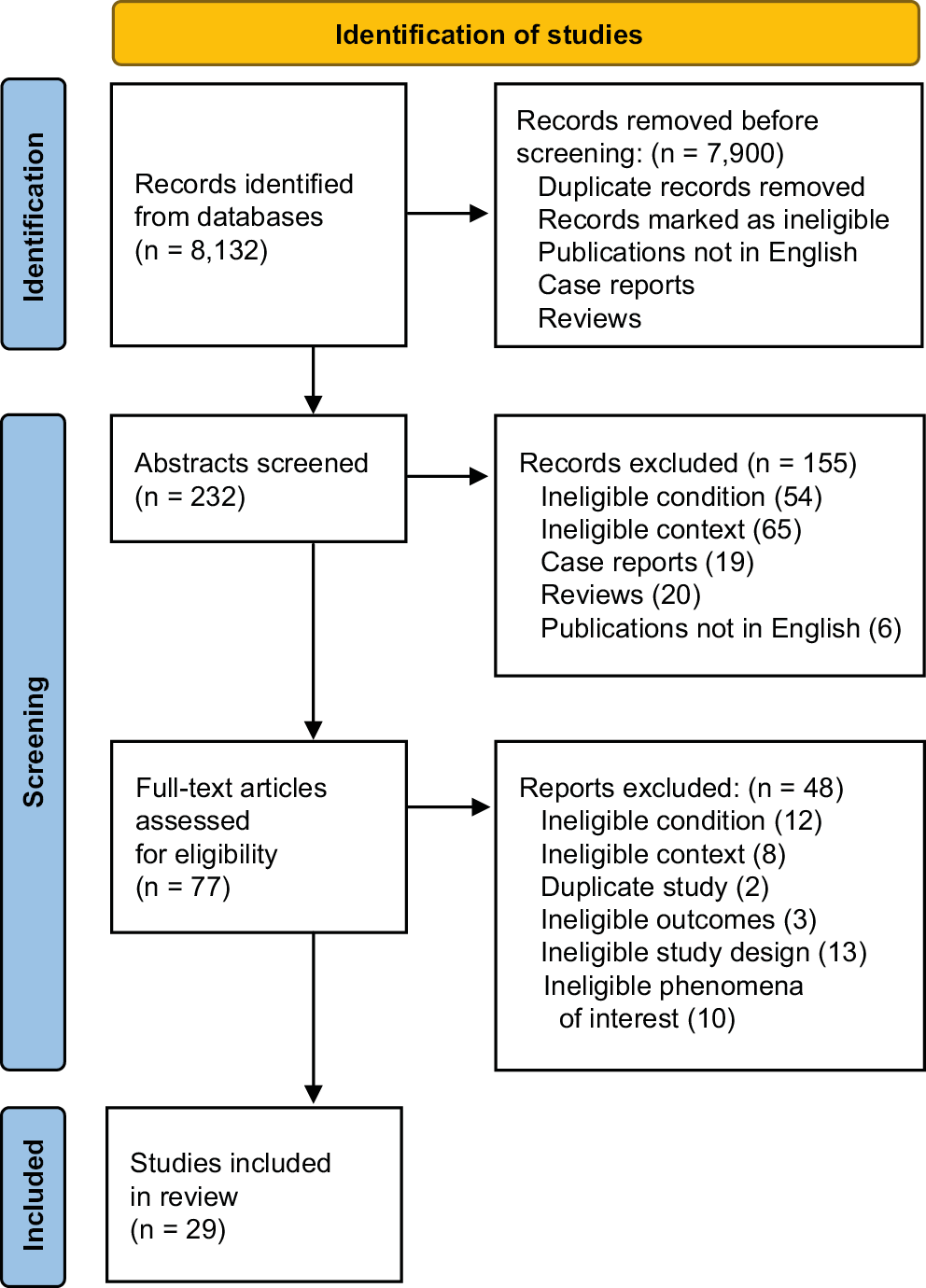
A systematic search was performed by screening the MEDLINE database. This database was searched using the terms “obstructive sleep apnea”, “OSAS, “CPAP” in conjunction with “otorhinolaryngological manifestation”, “ear”, “nose”, “throat’’, “oral cavity”, “pharynx”, “larynx”, “hearing”, “vertigo”, “head and neck cancer’’, “olfaction”, “voice”, “infection”, “sinusitis”, “tinnitus”, “tympanosclerosis”, “myringosclerosis”, “halitosis”, “epistaxis”, “candidiasis”, “xerostomia”, “taste”, and “facial pain”. Boolean operators (NOT, AND, OR) were also searched in succession to narrow and broaden the search. Articles that met the inclusion criteria were examined (Figure 1).
Inclusion and exclusion criteria
We included all studies published in English between January 1979 and March 2021 that addressed the links between OSAS, CPAP and otolaryngological manifestation. Obstructive sleep apnea syndrome had to be diagnosed by polysomnography. There were no age restrictions.
Due to the focused scope of this review, ENT symptoms in conditions leading to OSAS, such as obesity, hypertension and facial deformities, were not discussed. The search was limited to publications in English. Articles that did not cover the topic areas, case reports, reviews, and duplicate articles were excluded.
Data extraction and assessment of bias risk
The title, abstract, keywords, authors’ names, journal names, and year of publication of the identified records were exported to a Microsft Excel spreadsheet (Microsoft Excel 2023; Microsoft Corp., Redmond, USA). Two independent reviewers (J.C. and K.S.) screened the titles and abstracts of the records, and papers that clearly did not address the topic areas were discarded. Then, the 2 reviewers performed an eligibility assessment by carefully screening the full texts of the remaining papers independently. During this phase, disagreements between the reviewers were discussed and resolved by consensus. In the event of disagreement, the views of a 3rd reviewer (M.F.) would have been considered.
Results
Study selection
The search resulted in 8,132 studies, of which 232 abstracts were reviewed. Of the abstracts screened, 54 were ineligible condition, 65 were ineligible context, 19 were case reports, 20 were reviews, and 6 were not in English. Seventy-seven studies were subject to a full eligibility assessment. Our review ultimately included 29 articles; 12 studies showed ear dysfunction, 11 described nose dysfunction and 6 showed pharynx dysfunction. Included articles described ENT symptoms of hearing dysfunction, vestibular function disorders, cerebrospinal fluid leak, Eustachian tube (ET) dysfunction, rhinosinusitis, olfaction and taste disorders, dysphagia, dry mouth symptom, and gastroesophageal reflux disease (GERD). A summary of the included studies and quality of ENT symptoms in OSAS and relevant findings are presented in Table 1 and Table 2.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31
Risk of bias in studies
To evaluate the quality of the identified studies, a tool proposed by Kmet et al.32 has been applied separately for qualitative and quantitative studies. This instrument for quality assessment takes into consideration the following criteria: the precision of the research aims; the report of study design, materials and methods, justification of study’s relevance, sampling strategy, and reflexivity. Each component was rated using a 3-point response scale (2 points for “yes”, 1 point for “partial” and 0 points for “no”). If the criterion was not applicable for a study, then its score was excluded from the computation of the overall score. From the 5 possible cutoff points proposed by Kmet et al.32 (75%, 70%, 65%, 60%, and 55%), the 65% threshold was selected to indicate moderate quality. Overall, the quality of the 29 selected studies was evaluated. All of the studies met the 65% threshold and were included in the analyses.
Discussion
Ear
Hearing dysfunction
The cochlea is particularly sensitive to low partial pressure of oxygen (pO2) due to its high energy demands, making ischemic hypoxia a significant factor in sudden hearing loss.3 It has been shown that OSAS reduces blood pO2 levels, adversely affecting cochlear hair cells and central auditory structures, including the auditory pathway. A study by Li et al. demonstrated high-frequency hearing loss in pure-tone threshold audiometry among patients with severe OSAS.3 Similar findings were observed in patients with moderate OSAS, who also exhibited deteriorated speech discrimination.4, 5 Pure-tone thresholds and speech discrimination thresholds correlated positively with apnea–hypopnea index (AHI) and desaturation index, and negatively with minimum O2 saturation (p < 0.001).5 Notably, hearing impairment in the initial phase of OSAS may go unnoticed by patients and might not be detected through pure-tone audiometry. However, there is a significant reduction in the amplitude of the distortion product otoacoustic emissions (DPOAE).3 The DPOAE, an objective hearing test commonly used in clinical settings to assess the health of the inner ear (outer hair cells), shows changes in patients with OSAS as an early indicator of cochlear damage before it manifests itself in reduced hearing thresholds. In the study, 71% of OSAS patients showed abnormal DPOAE results at 750 Hz, compared to 0% in the control group (p < 0.001). Similarly, abnormalities were observed at other frequencies: 47% at 1 kHz, 26% at 2 kHz, 33% at 4 kHz, 28% at 6 kHz, and 34% at 8 kHz, while no abnormalities were detected in the control group (p < 0.001 for all frequencies).3 Damage to hair cells at the cochlear base, where higher frequencies were encoded according to tonotopy, was more pronounced, which explained the observed deterioration in high-frequency hearing in pure-tone audiometry.6 Additionally, 42% of OSAS patients experience tinnitus within the 4–8 kHz frequency range, further confirming the frequent damage to hair cells at the cochlear base.5
Another objective hearing test that exhibits abnormalities in patients with sleep apnea is the auditory brainstem response (ABR).3 The ABR assesses the accuracy of transmission from the auditory nerve to the auditory radiation in the forebrain, with results consisting of 7 waves, labeled I–VII, whose latencies, interlatencies and morphology are analyzed. At a stimulation rate of 11 times per second, the binaural wave I latency in OSAHS patients was 1.51 ±0.13 ms, compared to 1.33 ±0.07 ms in the control group (p < 0.001). The wave V latency in OSAHS patients was 5.65 ±0.23 ms, compared to 5.53 ± 0.23 ms in the control group (p = 0.0016). At the higher stimulation rate of 51 times per second, the wave I latency was 1.64 ±0.12 ms in OSAHS patients compared to 1.44 ±0.06 ms in the control group (p = 0.0001). The latency of wave V was 5.92 ±0.26 ms in the OSAHS group compared to 5.80 ±0.18 ms in the control group (p = 0.0077).3 Li et al. reported increased wave I and V latencies in patients with OSAS, indicating conduction disturbances in the central auditory pathway, which was corroborated by İriz et al. through speech discrimination tests.3, 7 While Li et al. demonstrated increased wave I and V latencies, they also noted that air-conduction thresholds in patients with moderate-to-severe OSAHS were similar to those of healthy controls. This suggests that while central auditory pathways are affected, peripheral hearing remains relatively unaffected.3
Peripheral vestibular system damage
Obstructive sleep apnea syndrome, by affecting the inner ear, also disrupts the peripheral vestibular system, which is crucial for maintaining balance, stability and spatial orientation. The primary symptoms of peripheral vestibular system damage include vertigo and nystagmus, accompanied by visceral autonomic symptoms such as nausea and vomiting. The primary test used to assess labyrinthine function is videonystagmography (VNG), which reveals a significantly higher occurrence of nystagmus and canal paresis in patients with moderate-to-severe OSAS compared to those with mild OSAS.8 Furthermore, patients with moderate-to-severe OSAS exhibit higher scores on the Dizziness Handicap Inventory (DHI) survey, indicating the prevalence and impact of dizziness on daily life.8 Micarelli et al. demonstrated vestibular dysfunction in patients with no evidence of vestibular dysfunction on otologic examination using the vestibulo-ocular reflex (VOR), which stabilizes gaze during head movements by activating the vestibular system. In the video Head Impulse Test (vHIT), the mean VOR gain was significantly lower in OSA patients (0.42 ±0.06) compared to healthy controls (1.05 ±0.16).9 This masking of clinical evidence of vestibular disorder can be explained by the central vestibular system compensating for disequilibrium caused by peripheral vestibular system damage.9 Impaired vestibular function should prompt physicians to refer patients for polysomnography, especially when the etiology of vestibular organ damage is not fully understood.
Cerebrospinal fluid leak
The etiology of cerebrospinal fluid leak (CSFL) is often unclear and frequently cannot be identified. The most common cause is trauma to the dura mater due to surgery or accidents. However, this condition may also be associated with OSAS, where it is linked to increased intracranial pressure (ICP). Apneic episodes can lead to hypoxia and hypercapnia, resulting in increased cerebral blood flow and elevated ICP.10 Additionally, OSAS frequently coexists with obesity, a potential risk factor for spontaneous CSF (sCSF) leak, as obesity can lead to idiopathic intracranial hypertension (IIH).10, 11 Consequently, weight loss may reduce both OSAS severity and the incidence of elevated ICP.11 However, increased ICP in OSAS patients is independent of concurrent obesity and is correlated with apneic episodes and decreased SaO2 levels.10 In this context, Yancey et al. noted that 47% of sCSF patients had OSAS, suggesting that polysomnography (PSG) should be considered in patients with sCSF.11
Eustachian tube dysfunction
Maintaining normal middle ear pressure is vital to middle ear health and depends on the proper functioning of the ET. Obstructive sleep apnea syndrome has been identified as an independent risk factor for ET dysfunction.12 Negative pressure in the upper airway of OSAS patients may lead to tissue collapse around the ET, resulting in dysfunction. Eustachian tube dysfunction causes negative middle ear pressure (MEP) and may contribute to various otologic conditions such as conductive hearing loss, tympanic membrane retraction and chronic otitis media.13 Continuous positive airway pressure therapy provides a pneumatic stent for the upper airway and also prevents the collapse of the ET ostia, thereby reducing the negative consequences for the middle ear. Thom et al. noted that the average MEP during sleep in CPAP users increase to more than half its value, thus mitigating the effects of decreased pressure in the middle ear.13 This relationship was further supported by Sivri et al., who observed a conversion from a type B or C (abnormal MEP) tympanogram to a type A (normal MEP) in a number of CPAP users.14 Continuous positive airway pressure therapy can also be successfully used in tympanic membrane retraction.13 However, increases in MEP in CPAP therapy can cause otalgia, ear fullness, pharyngitis, and in rare cases even tympanic membrane rupture, pneumocephalus and tension pneumocranium.13
Nose
Rhinosinusitis
One of the most important functions of the nasal mucosa is mucociliary clearance, which cleanses the sinuses. In the severe OSAS group, Deniz et al. found that the mean mucociliary clearance time was significantly prolonged.15 These findings suggest that OSAS may lead to deterioration of mucociliary transport, further causing chronic rhinosinusitis (CRS).15 The impact of CPAP on mucociliary transport is significant. Some studies showed no adverse effect of CPAP therapy on mucociliary function, while others revealed negative changes in the nasal epithelium caused by dry and cold air.16, 17 Reported adverse effects of CPAP include epithelial flattening, decreased cilia number and prolonged mucociliary clearance. Cold and dry air provokes the release of inflammatory factors, causing inflammation of the nasal mucosa.17 However this study used non-humidified CPAP, whereas humidified CPAP is now the standard, as it redusces negative side effects.
Symptoms such as fatigue, difficulty sleeping, nasal obstruction, facial pain, and headaches are commonly reported by OSAS patients and can mimic CRS symptoms.18 The 22-item Sinonasal Outcome Test (SNOT-22) is commonly used in patients with CRS. Ji et al. showed that SNOT-22 is a valuable tool for identifying undiagnosed OSAS.18 Their study concluded that OSAS patients more often cited sleeping difficulty as the most troublesome symptom compared to CRS patients, who reported nasal and ear-related complaints (thick nasal drainage, ear pain and anosmia). Therefore, patients with high sleep -and psychological domain scores on SNOT-22 should undergo PSG.18
Pediatric rhinosinusitis may also be associated with OSAS. According to Arens et al., OSAS in children leads to sinus inflammation, particularly in the maxillary sinus.19 However, in most cases, pediatric OSAS occurs due to adenoid hypertrophy, which itself generates inflammation, defects in mucociliary clearance and nasal obstruction.
Olfaction and taste disorders
Similar to hearing, the sense of smell is sensitive to hypoxia. This sensitivity is evident in studies showing that the cholinergic neurotransmitter system is susceptible to hypoxemia, affecting brain activity in the thalamus, hippocampus, prefrontal, and posterior parietal cortex.20, 21 These neuroanatomical structures influence cognitive abilities and may be implicated in dysfunctional smell identification and odor differentiation.20 Liu et al. demonstrated significant reductions in odor thresholds, odor discrimination (OD), odor identification (OI), and total taste score in patients with snoring and OSAS in subjective odor and taste measurements.22
Sleep apnea has been shown to increase pro-inflammatory effects on the nasal mucosa by markers such as interleukin (IL)-8 and tumor necrosis factor alpha (TNF-α).20 Inflammation of the olfactory epithelium disrupts odor transmission in the olfactory nerve.
Numerous clinical studies have confirmed that CPAP therapy can improve olfaction in patients with OSAS through several mechanisms.20, 23, 24 First, by increasing oxygen saturation, which has a positive effect on cognitive function and by normalizing cholinergic neurotransmitters.20, 23 Second, positive airway pressure reduces the amount of inflammatory factors in the airways, which improves the condition of the olfactory epithelium and improves odor identification.20, 23
Taste in OSAS patients may also be affected by neuropathy resulting from vibrations in the upper respiratory tract tissue. However, anosmia itself also impairs the gustatory sensations.25
Pharynx
Dysphagia
Dysphagia frequently occurs in patients with OSAS.26 The fiberoptic endoscopic evaluation of swallowing (FEES) has revealed swallowing abnormalities that were not self-reported by patients.26 During the FEES, a flexible endoscope records the swallowing process using boluses of different textures (thin liquid, semisolid and solid) and volume to assess the signs and symptoms of oropharyngeal dysphagia.26 The FEES exam demonstrated that 28% of patients had piecemeal deglutition with the 10 mL liquid trials and 64% had spillage with the 20 mL liquid trials, confirming dysphagia.26 Furthermore, another study indicated a disturbance in the protective role of the epiglottis.27 In half of the study participants with OSAS, the epiglottis remained elevated and the airway open after taking a bolus of food, indicating an increased risk of aspiration when the patient inhaled rapidly or was speaking at the same time.27 However, the severity of dysphagia did not correlate with the severity of OSAS.26
Vibration trauma during snoring in OSAS causes local neuronal damage, resulting in failure of the swallowing reflex response. A larger volume of food in the pharynx is required to provoke this reflex compared to healthy patients.26, 28 Heiser et al. showed a decreased mechanical sensitivity of the pharynx using a 2-point discrimination test at the soft palate and air puffs at the posterior pharyngeal wall in OSAS patients.29 This research also found impaired chemosensitivity of the pharynx during stimulation with capsaicin and CO2 at the posterior pharyngeal wall, which suggest a possible link between local neuropathologies and OSAS.29
Dry mouth symptom
Saliva performs essential functions such as protecting dentition and the oral mucosa, aiding digestion, providing antibacterial activity, enhancing taste, and lubricating oral tissues. Studies have found that approx. 74% of OSAS patients report dry mouth and the necessity for water intake during the night or immediately upon waking.30 Moreover, dry mouth may reduce the function of taste receptors.25
Other issues reported by OSAS patients include painful burning and/or tingling sensations of the oral mucosa, lips, tongue, gingiva, and teeth, as well as difficulties with chewing, halitosis and dental impairment. Halitosis and dental impairment may also be associated with laryngopharyngeal reflux, which is more common in OSAS.33
Gastroesophageal reflux
Some studies have noted a reduction in OSAS symptoms during GER treatment and an improvement in GER symptoms during OSAS treatment.31 Continuous positive airway pressure therapy has also been observed to decrease nocturnal GER symptoms.31 Green et al. suggested that OSAS may lead to GER, rather than GER causing OSAS, though the direction of causality remains controversial.31
Laryngopharyngeal reflux (LPR) is clinical entity different from classic GER.31 The main symptoms of LPR are dysphonia, chronic cough, sore throat, and pharyngeus globus.31 These symptoms are caused by the irritating effect of acid which can affect tissue superior to the hypopharynx and larynx. Obstructive sleep apnea syndrome can induce GER and LPR by creating greater negative intrathoracic pressure, thereby impairing esophageal sphincter function.31 Gastric acid and other gastric contents, such as pepsin, cause inflammation, hypertrophy and sensory disturbances in the larynx and pharynx that contribute to the progression of OSAS.31 Given that the associations between GER and OSAS are still unclear, further clinical trials are necessary to elucidate their respective mechanisms.
Limitations
This study has several limitations that should be acknowledged:
1. Language restriction: Only articles published in English were included, potentially excluding relevant studies in other languages.
2. Search limitation: The search was conducted only in the MEDLINE database, which may have resulted in the omission of relevant studies available in other databases.
3. Exclusion of certain conditions: ENT symptoms related to conditions leading to OSAS, such as obesity, hypertension and facial deformities, were not discussed, which might have provided a more comprehensive understanding of the topic.
4. Quality of included studies: While all included studies met the 65% quality threshold, varying levels of quality and potential biases within these studies could influence the overall findings of this review.
Conclusions
This paper has established that otolaryngologic manifestations are common symptoms of OSAS, particularly in severe cases. It is often the case that ENT symptoms, which include hearing dysfunction, rhinosinusitis, olfaction disorders, and gastroesophageal reflux, are linked with OSAS. This can result in significant complications. Recognizing these symptoms is crucial for differential diagnosis in patients presenting with sleep-related issues. The presence of these manifestations should prompt the use of polysomnography to confirm an OSAS diagnosis, especially when they occur with other sleep disorder symptoms. Continuous positive airway pressure therapy, the primary treatment for OSAS, has been shown to alleviate many ENT symptoms associated with the condition. Future research should focus on further elucidating the connections between ENT symptoms and OSAS to enhance diagnostic accuracy and treatment outcomes. This will underscore the role of otolaryngology in managing this pervasive disorder.