Abstract
Background. Mitochondrial dynamics is an important field in cell biology, encompassing mitochondrial fission and fusion. The balance between fission and fusion is responsible for the stability of the mitochondrial network and can be a regulator of mitochondrial function. Recent studies have emphasized that an imbalance in mitochondrial dynamics is the root cause of dysfunction and is involved in various stages, such as oxidative stress, inflammation and apoptosis. Reversing this imbalance can effectively alleviate disease conditions. Although the importance of mitochondrial dynamics has been widely recognized, there is still a lack of literature on the qualitative and quantitative description and analysis of advances in this field.
Objectives. This study is a bibliometric analysis of research trends, collaboration networks and thematic evolution in mitochondrial dynamics from 2000 to 2023.
Materials and methods. Using the Web of Science Core Collection (WoSCC) database, we performed a bibliometric review, applying VOSviewer and CiteSpace to visualize and analyze publications, citations, collaborations, and key word trends.
Results. We analyzed 332 publications, identifying China and the USA as leaders in research output and international collaborations. Significant contributions were made by institutions like Chiang Mai University and the California Institute of Technology (Caltech), with major research shifts from basic mitochondrial functions to roles in diseases like Alzheimer’s and cardiovascular disease.
Conclusions. Mitochondrial dynamics research has expanded, with increasing attention to its role in disease mechanisms. Future research should further explore these connections, potentially leading to innovative treatments.
Key words: mitochondrial dynamics, bibliometrics, VOSviewer, CiteSpace, Science Citation Index
Introduction
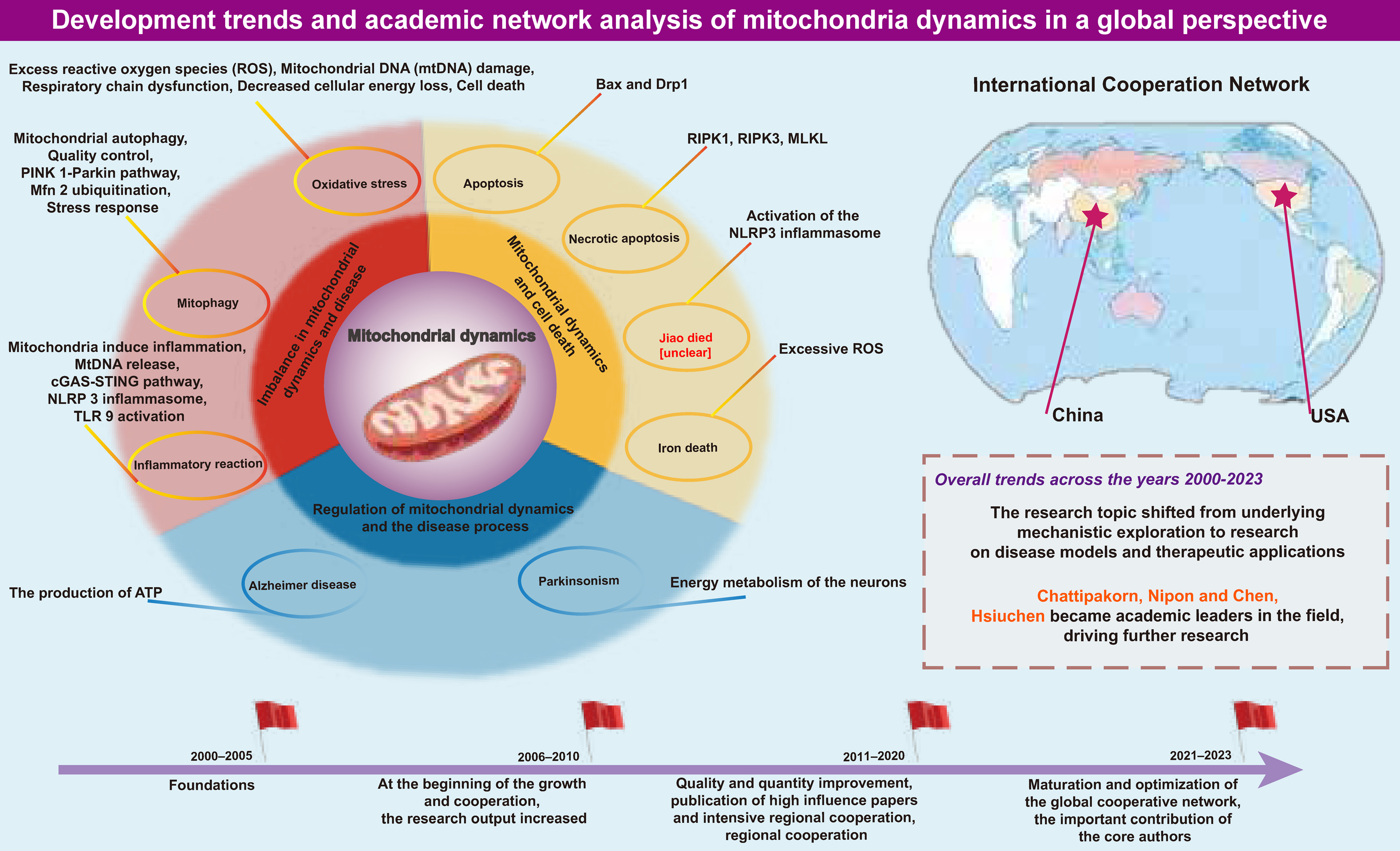
Mitochondrial dynamics, a field within cell biology focused on the morphological variability of mitochondria, primarily encompasses mitochondrial fission and fusion. In 1898, Benda first observed the morphological diversity of mitochondria, which can be presented in either spherical or filamentous shapes.1 Subsequently, in 1914, Lewis and Lewis discovered that mitochondria could change from one form to another following fission or fusion, thereby establishing the fundamental concept of mitochondrial dynamics.2 Dynamin-related protein 1 (Drp1) is the principal protein mediating mitochondrial fission, recruited to the outer mitochondrial membrane (OMM) by mitochondrial dynamics proteins 59 (MiD59) and MiD50, where it aggregates to form interlocking structures.3 In conjunction with fission 1 (Fis1) on the OMM, these aggregates constrict and sever the mitochondrion, producing 2 separate entities. Beyond transporting intramitochondrial components, fission is vital for isolating damaged mitochondrial segments to maintain a healthy mitochondrial network, which is crucial in the process of mitochondrial autophagy.4 Mitochondrial fusion, divided into OMM fusion and inner mitochondrial membrane (IMM) fusion, is mediated by mitofusion 1/2 (Mfn1/2) and optic atrophy 1 (Opa1), respectively. Fusion allows mitochondria to share components, compensating for each other’s deficiencies. Contrarily, the enhanced oxidative phosphorylation (OXPHOS) levels in a fused state aid in maintaining energy output under cellular stress. The balance between fission and fusion governs the stability of the mitochondrial network and dictates mitochondrial function.5 Recently, mitochondrial dysfunction has been implicated in the pathogenesis of various diseases, making mitochondrial dynamics an emergent research focus. Imbalances in mitochondrial dynamics are at the root of dysfunction, contributing to oxidative stress, inflammation and apoptosis; rebalancing these dynamics may potentially alleviate these conditions.
For instance, in neurodegenerative diseases such as Alzheimer’s disease (AD), the application of interventions that improve mitochondrial dynamics, such as antioxidants, has been shown to improve cognitive function in patients.6 Mitochondrial dysfunction, such as oxidative stress, can stimulate the release of neurotransmitters from neurons, thus playing a protective role for neurons.7 Mitochondrial dynamic imbalances often accompany disease states, which lead to mitochondrial structural disarray, contributing to disease onset.8 Mitochondrial dynamic dysfunction causes abnormal oxidative stress in neurodegenerative diseases, increasing free radicals, which leads to neuronal changes, such as reduced membrane permeability and decreased neuronal excitability, making it an important therapeutic target.9, 10 Additionally, mitochondrial dynamics play a critical role in synaptic function, neurotransmitter release and axonal transport. For example, the activation of mitochondrial fission promotes mitochondrial fragmentation in the medial prefrontal cortex, leading to mitochondrial dysfunction, impairing excitatory synaptic transmission and contributing to stress-related depressive-like behaviors.11 When quality control proteins like PINK1 in mitochondria are dysregulated, it leads to fewer axonal vesicles, abnormal synaptic connections and reduced neurotransmitter release, all of which are essential for neural circuit formation and synaptic efficacy.12 Hence, mitochondrial dynamics is considered a promising research target.
Objectives
Bibliometric analysis is a method that qualitatively and quantitatively describes and analyzes the progress of a particular discipline or research area. With modern technology, results can be visualized using knowledge maps, making the outcomes more comprehensive, aiding in data interpretation and revealing inherent connections between pieces of information. To our knowledge, no bibliometric analysis of mitochondrial dynamics has been conducted so far. Therefore, we undertook a systematic analysis to explore the state and trends of mitochondrial dynamics research from 2000 to 2023.
Materials and methods
Data collection and analysis of mitochondrial dynamics research: A comprehensive search based on the WoSCC database
This study sourced data from the Web of Science Core Collection (WoSCC) database as of June 19, 2023. The WoSCC, widely regarded as the most authoritative database in bibliometrics, encompassing the Science Citation Index Expanded (SCIE) and the Social Sciences Citation Index (SSCI). To delve into the latest research trends in mitochondrial dynamics, we employed a precise search formula: [TS = (mitochondrial dynamics)] AND [Publication Type = (article)] AND [Language = (English)]. Considering that English literature published in English is more likely to be recognized and evaluated by the international academic community due to global usage of this language, we have added a language filter to the search strategy. This search spanned from 2000 to 2023 (Table 1), with the goal of comprehensively capturing scientific advances in mitochondrial dynamics during this period.
The retrieved data included various information such as titles, authors, keywords, abstracts, institutions, countries, languages, and cited references. All these data were downloaded as “full records and cited references” for subsequent in-depth analysis and research.13 This study aimed to identify research hotspots and trends in mitochondrial dynamics and analyze the contributions and collaboration patterns of different countries and institutions in this field.
Bibliometric analysis of mitochondrial dynamics research using VOSviewer and CiteSpace tools
In the data analysis phase, the filtered data were imported into 2 specialized bibliometric analysis and visualization tools, VOSviewer v. 1.6.19 (Centre for Science and Technology Studies, Leiden University, Leiden, the Netherlands) and CiteSpace v. 6.1.R3 (College of Computing & Informatics, Drexel University, Philadelphia, USA), which employ a probability-based data normalization method and offer a variety of display methods. These visualization methods, based on key word analysis and co-authorship networks, aid in gaining a deeper understanding of the research trends and critical nodes in mitochondrial dynamics.
CiteSpace 6.1.R3 is an application based on set theory data normalization methods. It provides a visual overview of the research progress in mitochondrial dynamics by analyzing the similarity of the most strongly cited burst keywords extracted from the research. Additionally, CiteSpace can identify current research frontiers in the field, offering valuable insights for future research directions.13, 14, 15 To gain a deeper understanding of the global research landscape, we also employed the dual-map analysis method to examine the scientific citation network in mitochondrial dynamics worldwide. This approach visualizes the citation patterns and information flow between different academic disciplines, helping to reveal the interdisciplinary nature of mitochondrial dynamics research and the extent of international collaboration. This analysis provides a panoramic view of the core nodes in the academic network and their influence.
The combined use of these 2 tools reveals the core themes and trends in mitochondrial dynamics research and displays the dynamic changes in academic collaboration networks and research hotspots. Such analysis enables a deeper understanding of the research field’s development trajectory, providing valuable references and guidance for future studies. These results are crucial in discussing how to promote academic exchange and collaboration in the field of mitochondrial dynamics and how to effectively share research findings globally.
Results
Trends and future prospects in mitochondrial dynamics research
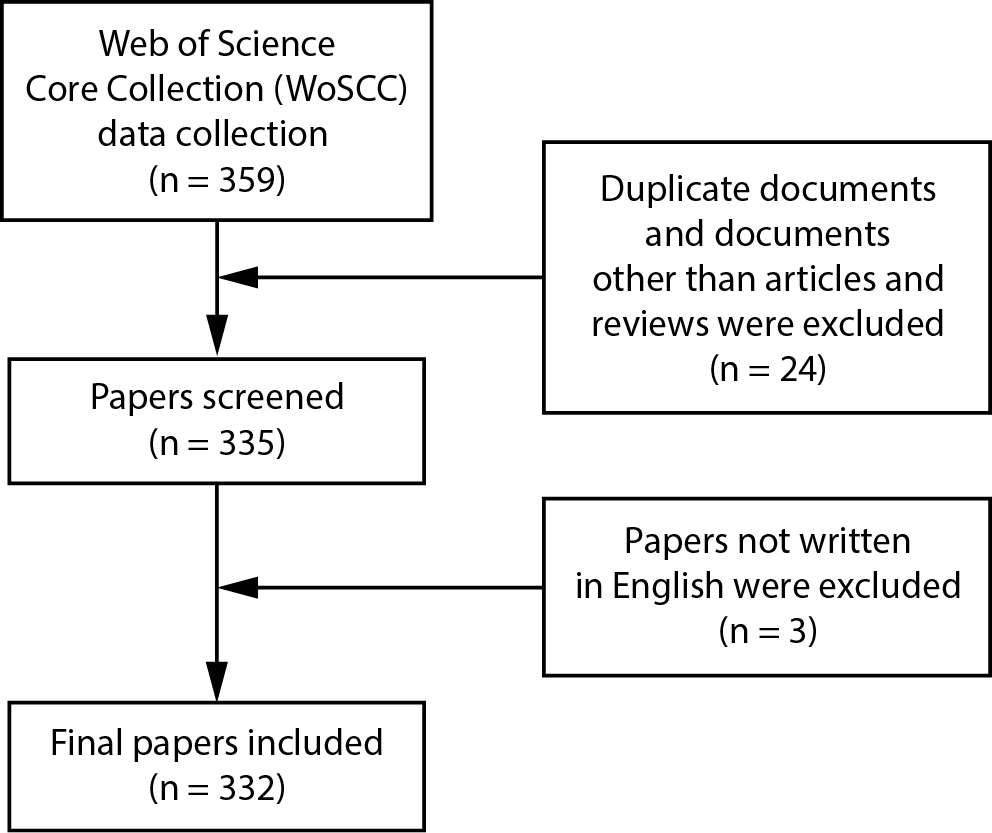
After an extensive search of the WoSCC database, 359 papers were initially identified. Following further screening and evaluation, 332 articles were ultimately included in this study (Figure 1). We found that the total number of articles related to mitochondrial dynamics has shown an upward trend (Figure 2). It reflects the growing emphasis on scientific research in this field and indicates that there may be more breakthroughs and discoveries in the future. Moreover, this rising trend may also suggest that the research foundation in this area is being strengthened, the research community is expanding, and research methods and technologies are continuously advancing.
We also observed a decline in the number of articles in 2018 and 2023 (Figure 2), which could be attributed to various factors, such as fluctuations in research funding, changes in science policies or a shift in research interests. Notably, the final publication count for 2023 is expected to increase, as the data currently cover only the 1st half of the year. Therefore, research on mitochondrial dynamics will continue in the coming years, yielding more academic contributions and clinical applications.
International collaboration and academic contributions in global mitochondrial dynamics research
In this study, 332 papers from 40 countries were analyzed. Table 2 lists the top 10 countries with the most published papers. In mitochondrial dynamics, China leads in publication quantity and citation count, followed by the USA and Italy, which also boast significant achievements (Figure 3). However, the average citation count for the USA and Italy exceeds 40, approximately double that of China. The UK also achieved an average citation count of 29.57, indicating a higher quality of scientific research in these countries.
Further analysis involved 17 countries with more than three publications, showcasing their publication distribution (Figure 4, Figure 5) and the strength of collaboration between countries (Figure 6). Collaborations in mitochondrial dynamics are primarily between China and the USA, with other countries needing to strengthen their collaborative ties.
These data reveal the global development and scientific collaboration networks in mitochondrial dynamics research. While China dominates in publication volume, the high citation counts of the USA and Italy reflect their research quality and international influence. Despite fewer publications, other countries, such as the UK, also show significant impact through high average citation counts.
Overall, these findings suggest that while countries vary in their contributions to mitochondrial dynamics research, further strengthening international cooperation and exchange is vital to advancing the field. Enhanced collaboration among nations can foster knowledge sharing, technological exchanges and innovative ideas.
Global institutional distribution and collaboration trends in mitochondrial dynamics research
In this study, 482 institutions contributed to the publication of 332 articles. Table 3 lists the top 10 institutions by the number of published articles and citation counts. Chiang Mai University (Thailand) leads with 16 publications, followed by the Chinese Academy of Sciences (CAS). North Carolina Central University (USA) and several Chinese universities, such as Ningxia Medical University and Shanghai Jiao Tong University, have significantly contributed to mitochondrial dynamics research. The CAS has the highest average citation count, indicating its papers’ exceptional scientific value. Since mitochondrial dynamics is a relatively new research area, we have yet to detect strong collaboration among institutions (Figure 7). Closer collaboration is observed among Chinese universities, likely due to geographical proximity.
These findings reflect the expanding global reach of mitochondrial dynamics research, with varying contributions from institutions worldwide. The prominent roles of Chiang Mai University and CAS and other Chinese universities’ active participation demonstrate Asia’s significant impact and influence in this research area. In particular, the high citation rate of CAS highlights its leadership in scientific research and academic guidance.
Moreover, the study reveals a notable trend: despite being an emerging research field, tight collaboration among institutions has yet to be become prominent. It may be due to the development stage of this field, with institutions still exploring and establishing collaboration networks. Therefore, promoting international and cross-institutional collaboration, especially in knowledge sharing and resource integration, is crucial for advancing the field of mitochondrial dynamics. As this area of research evolves, we anticipate more transnational and cross-institutional collaborations, leading to innovative outcomes and academic progress in this area.
Core authors and their academic impact on mitochondrial dynamics research
The 332 articles analyzed in this study encompass 2,227 authors (Figure 8). Following Lotka’s law,16 authors with more than 2.8 publications were defined as core authors (Figure 9, Figure 10), with the top 10 listed in Table 4. Notably, Nipon Chattipakorn from Chiang Mai University top the list with 16 publications, followed by Siriporn Chattipakorn (15 papers) and Andy P. Li (9 papers). It aligns with the results in Table 3, underscoring the significant influence of Nipon Chattipakorn and his team in the field of mitochondrial dynamics (Figure 9).
Co-cited authors are those cited by at least 2 authors simultaneously. Among the 12,988 co-cited authors, Hsiu-Chen Chen leads with 116 citations (Supplementary Fig. 1,2). Only this author had more than 100 citations in this emerging research area (Table 4).
These data indicate a group of core authors driving the research frontier in mitochondrial dynamics. The significant achievements of Nipon Chattipakorn and his team at Chiang Mai University highlight their vital role in the field. Meanwhile, Hsiu-Chen Chen, as the most co-cited author, has widespread influence and recognition in mitochondrial dynamics research (Figure 10).
These findings not only showcase the core academic strengths in mitochondrial dynamics but also identify the primary sources of knowledge dissemination and academic impact. As research deepens, these core authors and widely cited studies are expected to continue playing a pivotal role in driving the development of mitochondrial dynamics research. It also emphasizes establishing broader academic exchanges and collaboration networks to foster knowledge sharing and innovation.
Extensive distribution and impact of mitochondrial dynamics research in academic journals
Our study identified 332 articles on mitochondrial dynamics published across 198 academic journals (Supplementary Fig. 3,4). Table 5 lists the top 10 journals by the number of articles in mitochondrial dynamics, with Oxidative Medicine and Cellular Longevity and Frontiers in Pharmacology leading with 10 articles, followed by International Journal of Molecular Sciences with 9 articles.
Furthermore, these 332 papers collectively cited 2,377 journals, with the top 100 most-cited journals displayed in Supplementary Fig. 5,6. Table 6 lists the top 10 co-cited journals, including prestigious publications like Cell, Nature and Science. Notably, up to 90% of these top co-cited journals are ranked in the 1st quartile (Q1) of the Journal Citation Reports (JCR).
These data indicate that the academic outputs in mitochondrial dynamics are widely distributed across various scientific journals, encompassing multiple disciplines and research directions. Journals such as Oxidative Medicine and Cellular Longevity, Frontiers in Pharmacology and International Journal of Molecular Sciences significantly advance research, with numerous influential articles published.
The co-cited journals reveal the close association of mitochondrial dynamics research with other areas of life sciences, particularly in top-tier Q1 journals like Cell, Nature and Science. Their high citation counts underscore the importance and influence of mitochondrial dynamics research in life sciences, reflecting its wide recognition and esteem within the scientific community.
Mitochondrial dynamics research is published in multiple high-impact scientific journals, significantly contributing to knowledge dissemination and scientific communication within the field. As research progresses, this field will continue to showcase its findings in top-tier scientific journals, thereby advancing development and progress in the broader field of life sciences.
Analysis of key literature and future trends in mitochondrial dynamics research
Among the 332 articles on mitochondrial dynamics, the most cited is “Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling” (450 citations),17 followed by “Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury” (282 citations)18 and “Disruption of renal tubular mitochondrial quality control by myo-inositol oxygenase in diabetic kidney disease” (187 citations)19 (Table 717, 18, 19, 20, 21, 22, 23, 24, 25, 26; Supplementary Fig. 7,8). These highly cited papers primarily focus on the vital roles of mitochondria in life activities such as energy production, metabolism, apoptosis, and intracellular signaling, as well as the impact of mitochondrial dynamics imbalance in diseases like acute kidney injury, neurodegenerative disorders, diabetes, and obesity.20, 21, 22, 23, 24, 25, 26
The relationships between co-cited references represent connections formed when an article published later cites 2 papers. VOSviewer visualizes co-cited references related to mitochondrial dynamics (Supplementary Fig. 9,10).26 “Mitochondrial fission, fusion, and stress” by Youle and Van Der Bliek, published in Science, is among the top 10 most cited references (Table 83, 4, 27, 28, 29, 30, 31, 32, 33, 34). As a critical review article, it elaborates on the definitions of the 2 main aspects of mitochondrial dynamics – fission and fusion – and details the mechanisms of these phenomena. Additionally, that paper discusses dynamic balance as a critical factor in maintaining mitochondrial network homeostasis under cellular stress: Fusion is impaired to prevent contamination of other mitochondria at a certain level of mitochondrial damage, and asymmetric fission is caused by uneven distribution of mitochondrial protein aggregates under cellular stress.
These analyses of highly cited literature and co-cited references reveal key research themes and trends in mitochondrial dynamics.3, 4, 27, 28, 29, 30, 31, 32, 33, 34 They showcase the critical role of mitochondria in cellular functions and diseases and highlight the importance of understanding the mechanisms of mitochondrial dynamic balance for future therapeutic strategies. With continued research, we expect more innovative findings to emerge, further propelling the progress of mitochondrial dynamics research in life sciences and medicine.
Key word analysis and research trends in mitochondrial dynamics
In this study of mitochondrial dynamics, 1,223 keywords were extracted from 332 articles. Six keywords appeared more than 50 times, including “oxidative stress”, “fission”, “apoptosis”, “fusion”, “mitochondrial dynamics”, and “mitochondria” (Supplementary Fig. 11,12; Table 9). The most frequently occurring key word was “oxidative stress” (121 times), followed by “fission” (100 times) and “apoptosis” (97 times) (Table 9). Table 10 lists the top 20 molecular-, pathological process- and disease-related keywords associated with mitochondrial dynamics. In terms of molecules, the top 3 were essential proteins regulating mitochondrial dynamics: Drp1 (42 mentions), Mfn2 (32 mentions) and Opa1 (21 mentions). The most commonly mentioned pathological processes included oxidative stress (121 mentions), fission (100 mentions), apoptosis (97 mentions), fusion (82 mentions), mitochondrial dysfunction (47 mentions), and autophagy (43 mentions). The most studied diseases in the field of mitochondrial dynamics included skeletal muscle disease (20 mentions), AD (14 mentions), cardiovascular diseases (13 mentions), Parkinson’s disease (13 mentions), and cancer (12 mentions).
The color changes in the overlay visualization exported from VOSviewer represent the average publication year (APY) (Supplementary Fig. 13). CiteSpace’s timeline view clusters evolving high-frequency keywords (Supplementary Fig. 14). The largest cluster is No. 0 (death), followed by No. 1 (apoptosis), No. 2 (reperfusion injury), and No. 3 (acute lung injury). Notably, key word clusters focused on detailed mechanisms, such as No. 0 (death) and No. 1 (apoptosis), have ceased in recent years, while others related to specific diseases, like No. 2 (reperfusion injury) and No. 3 (acute lung injury), continue to develop.
Additionally, we detected citation bursts to better understand the development of mitochondrial dynamics research (Supplementary Fig. 15). Citation bursts reflect the frequency of any key word over a specific time. Analysis of citation bursts not only focuses on the evolution of research hotspots but also summarizes recent research trends and suggests potential directions for future studies. The distribution of burst keywords is relatively even, with no year experiencing a sudden influx of burst terms. Opa1 exhibits the most robust burst (strength = 4.6), while axonal transport had the most extended burst duration from 2011 to 2017.
These analyses reveal the main foci and trends in mitochondrial dynamics research. The distribution of keywords indicates that pathological processes like oxidative stress, fission and apoptosis are current research focuses, while diseases related to mitochondrial dynamics, such as skeletal muscle disease and AD, are also receiving extensive attention.
Dual-map analysis of mitochondrial dynamics research
Supplementary Fig. 16 presents a dual-map overlay visualization analysis of research on cellular mitochondrial dynamics from 1956 to 2023. The dual-map analysis illustrates global academic collaboration and the knowledge flow patterns between disciplines. The research primarily focuses on molecular biology, medicine and chemistry. The close citation relationships between these fields reflect the active interdisciplinary collaboration network, with molecular biology and medicine playing a particularly dominant role in advancing mitochondrial dynamics research.
Based on the specific details of the dual-map analysis, the left and right sides, respectively, show the distribution patterns of citing journals and cited journals. The clustering of citing journals is mainly concentrated in the fields of “Medicine, Medical, Clinical,” “Biology, Molecular, Immunology” and “Physics, Materials, Chemistry,” indicating that articles on mitochondrial dynamics are primarily published in journals focusing on clinical medicine, biological sciences and chemistry. On the other hand, the cited journals show concentrations in “Molecular, Biology, Genetics,” “Health, Nursing, Medicine” and “Physics, Materials, Chemistry,” suggesting that research in this field relies on foundational discoveries in genetics, molecular biology and clinical studies. The citation paths between the left and right maps demonstrate the main citation flows from “Medicine, Medical, Clinical” to “Molecular, Biology, Genetics,” and from “Biology, Molecular, Immunology” to “Health, Nursing, Medicine.” This further reveals the close connection between clinical medicine and fundamental biological research in mitochondrial dynamics, underscoring the interdisciplinary nature of the field.
This dual-map analysis not only highlights the core research disciplines within the mitochondrial dynamics field but also reveals global trends in knowledge flow and interdisciplinary collaboration.
Discussion
The WoSCC database shows that between 2000 and 2023, 332 articles on mitochondrial dynamics were published in 198 academic journals, authored by 2,227 researchers from 482 institutions across 40 countries. This trend indicates the growing global interest and research in mitochondrial dynamics.
China’s contribution to mitochondrial dynamics research is particularly notable, leading the world in published papers. The performance of 8 high-output institutions, including the CAS, underscores the increasing emphasis on mitochondrial dynamics research in Chinese institutions. While the number of publications from the USA, Italy and the UK lags behind China, these countries’ articles garner more citations, indicating superior quality. However, the collaborative network among countries remains at a nascent stage. Aside from the close ties between China and the USA, connections among other countries are relatively sparse, suggesting a need for stronger international cooperation and more profound exchange to understand mitochondrial dynamics comprehensively.
Nipon Chattipakorn from Chaing Mai University is the most prolific author in this field, mainly researching the role of mitochondrial dynamics in myocardial ischemia/reperfusion (I/R) injury. His papers indicate that a single dose of melatonin, acute metformin, erythropoietin (EPO), and donepezil alleviate myocardial I/R injury, an effect achieved through the regulation of mitochondrial dynamics.35, 36, 37, 38 Hsiu-Chen Chen from the California Institute of Technology (Caltech) is the most cited author, focusing on the intrinsic mechanisms of mitochondrial dynamics, including molecular expression related to Drp1 recruitment (Fis1, Mff, MiD49, and MiD51), AMPK-mediated mitochondrial fission and mitochondrial heterogeneity and dysfunction caused by mitochondrial fusion disorders.3, 39, 40 Notably, a paper from 2003 by Chen et al. titled “Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development” is also among the top ten most-cited articles. This article highlights the crucial role of mitochondrial fusion in embryonic development, stating that fusion proteins MFN1 and MFN2 exist as homotypic or heterotypic oligomers, promoting mitochondrial fusion either cooperatively or independently, thus maintaining mitochondrial collaboration and protecting against respiratory dysfunction.28
Oxidative Medicine and Cellular Longevity published the most articles in the journal analysis. Additionally, co-cited journals include high-quality publications such as Proceedings of the National Academy of Sciences of the United States of America, Journal of Cell Biology, and the top-tier journals Cell, Science and Nature.
As previously mentioned, the cited references primarily discuss the impact of mitochondrial dynamics on specific disease types, while co-cited references focus more on analyzing molecular and cellular intrinsic mechanisms. Most cited references were published after 2015, while most co-cited references predate 2010, aligning with the research shift from mechanisms to disease. We observed a fluctuation in the publication volume of mitochondrial dynamics-related literature in 2018 and 2023. It may be due to the interdisciplinary integration of mitochondrial dynamics with other fields, such as bioinformatics and systems biology, leading researchers to publish their work in these emerging interdisciplinary areas, thus impacting the publication volume in the mitochondrial dynamics field. As research on mitochondrial dynamics progresses, the complexity and technical demands of the studies may increase, resulting in only laboratories equipped with specific technologies and resources being able to conduct relevant research, consequently affecting the overall publication volume.
Through key word iteration, research trends were mapped. Using overlay and timeline visualizations, we visualized the evolution of keywords. By analyzing key word co-occurrence (Table 8, Table 9), key word overlay and timeline (Supplementary Fig. 13,14), and key word bursts (Supplementary Fig. 15), we objectively assessed the hotspots and frontiers in mitochondrial dynamics research. These 3 aspects are summarized below.
Mitochondrial dynamics imbalance and disease: From oxidative stress to inflammation
An imbalance in mitochondrial dynamics is the fundamental cause of mitochondrial dysfunction, affecting various aspects such as oxidative levels, metabolic regulation, autophagy, and inflammatory responses. Our key word analysis revealed that early research predominantly focused on oxidative stress. Reactive oxygen species (ROS), representative components in oxidative stress, can be overproduced following an imbalance in dynamics. As typical byproducts of the electron transport chain (ETC), ROS are highly reactive superoxide anions with strong oxidizing properties that can damage proteins, lipids and DNA.34 Mitochondrial DNA (mtDNA) is especially susceptible to ROS-induced mutations and damage. The progressive accumulation of mutated mtDNA can lead to the loss of functional respiratory chain complexes, resulting in bioenergetic decline and cell death.41
Mitophagy, a frequently mentioned key word, is a protective mechanism for quality control by eliminating outdated or damaged mitochondrial components (Table 9). It has been established that mitophagy is necessary for fission.27 Dysfunctional components can be isolated and degraded through fission to compensate for nutrient scarcity. Furthermore, fusion proteins also participate in mitophagy. The PINK1/parkin pathway, known to mediate mitophagy, involves Mfn2, specifically colocalizing with PINK1 on the OMM during depolarization. Phosphorylated Mfn2 by PINK1 acts as a receptor to recruit activated parkin, prioritizing its ubiquitination.42, 43 Following Mfn2 ubiquitination, mitophagy initiators such as LC3 are recruited, initiating mitophagy.44 Additionally, Opa1 can be cleaved into 2 isoforms, long Opa1 and short Opa1 (L-Opa1 and S-Opa1). Under mitochondrial stress conditions, S-Opa1, derived from the hydrolysis of membrane-anchored L-Opa1, can be selectively used for mitophagy.45
Mitochondria-induced inflammation is a recent research interest. Upon injury, mitochondrial contents are released into the cytoplasm. Among these, mtDNA, a primary endogenous damage-associated molecular pattern (DAMP), induces inflammation through 2 main pathways due to its high specificity.46 In the 1st pathway, mtDNA is released into the cytoplasm through the permeability transition pore complex (PTPC) or pores formed by apoptotic regulators (Bax) and BCL2 antagonist/killer 1 (Bak1).47, 48 Cytosolic DNA sensor cGAS catalyzes the formation of cyclic dinucleotides (cGAMP) upon binding DNA, initiating inflammatory responses via cGAMP-interacting protein 1 (STING1) and subsequent synthesis of cytokines like interferon-β1 (IFN-β1), interleukin (IL)-6 and tumor necrosis factor (TNF). On the other hand, ROS-oxidized mtDNA can activate the NLRP3 inflammasome, promoting the secretion of downstream IL-1β and IL-18.49, 50 Additionally, mtDNA can endogenously bind toll-like receptor 9 (TLR9) in endosomes, activating the downstream classical nuclear factor kappa B (NF-κB) inflammatory pathway.51 Rodríguez-Nuevo et al. found that specific deletion of OPA1 in muscles leads to mitochondrial dysfunction and increased mtDNA content. Conversely, the depletion of mtDNA reversed the activation of the TLR9 and NF-κB pathways and the inflammatory response.52 Irazoki et al. also observed that acute downregulation of dynamin proteins (Mfn1, Mfn2, Fis1, Drp1) in myoblasts leads to mtDNA release and differential inflammatory responses: Inhibition of fusion proteins Mfn1 or Mfn2 triggers mitochondrial fragmentation and TLR9-dependent NF-κB activation, while inhibiting Drp1 or Fis1 causes mitochondrial elongation, accompanied by NF-κB-dependent and type I interferon inflammatory responses.53 Although dynamics imbalance leading to mtDNA extramitochondrial localization and triggering TLR9 or cGAS-dependent inflammation has been reflected in literature, more detailed mechanisms yet to be fully defined could be future research targets.
In addition to the mechanisms discussed above, aspects such as mitochondrial biogenesis, endoplasmic reticulum stress, calcium homeostasis, and protein phosphorylation have attracted widespread attention and are continually updated, warranting further exploration.
Mitochondrial dynamics and cell death: From apoptosis to the diversity of programmed cell death
In the field of mitochondrial dynamics, apoptosis is the most frequently mentioned form of cell death and has been extensively studied. The classic apoptotic pathway involves the complex of Bax and Drp1.54 Under mitochondrial stress, increased fission is accompanied by enhanced synthesis of the Bax-Drp1 complex. It leads to increased OMM permeability and cytochrome c release, activating caspase cascades and ultimately inducing apoptosis. Evidence suggests that defective mitochondrial fusion also responds to apoptotic signaling. Degradation or knockdown of Mfn2 enhances apoptotic intensity, while apoptosis in human retinal endothelial cells under hyperglycemic conditions is reversed following Mfn2 overexpression.55, 56, 57 Additionally, caspases activated during apoptosis are associated with organelle fragmentation and cristae remodeling. Opa1 maintains the integrity of cristae junctions, preventing cytochrome c release during apoptosis and thus exerting a protective function.58
Programmed cell death (PCD) has always been a widely studied field, encompassing necroptosis, pyroptosis and ferroptosis, in addition to apoptosis. Necroptosis mentioned in the keywords is an inflammatory PCD mediated by the activation of receptor-interacting protein kinase 1 (RIP1), receptor-interacting protein kinase 3 (RIP3) and mixed lineage kinase domain-like protein (MLKL), characterized by cell swelling, membrane rupture and release of cell contents. In a chronic kidney disease (CKD) rat model, increased expression of RIPK1, RIPK3 and MLKL along with Drp1 was observed, which was reversed after treatment with the RIPK1-targeting drug necrostatin-1 (Nec-1), suggesting excessive mitochondrial fission might be involved in necroptosis.59
Although pyroptosis and ferroptosis were not mentioned in the keywords, continuous research indicates potential links with mitochondrial dynamics. Pyroptosis is characterized by forming pores on the cell membrane and releasing cell contents, such as pro-inflammatory cytokines.60 The classic pathway of pyroptosis involves the activated NLRP3 inflammasome, which assembles and activates caspase-1 to cleave gasdermin D, a member of the gasdermin family, mediating membrane pore formation and enabling maturation and release of IL-1β and IL-18. Existing studies reveal connections between mitochondrial dynamics and the NLRP3 inflammasome. RNA virus infection mediates the assembly of the RIP1-RIP3 complex, activates Drp1, and then guides its mitochondrial translocation, excessive fission leading to mitochondrial damage, and NLRP3 inflammasome activation.61 CaMKII activation-induced Drp1 phosphorylation and mitochondrial fission produces excessive mitochondrial ROS (mtROS), driving NLRP3 inflammasome activation.62 Additionally, divalent manganese (Mn2+) inhibits Mfn2 expression, promoting mitochondrial superoxide generation and accumulation and triggering NLRP3 inflammasome activation in microglia.63
Ferroptosis is a PCD characterized by excessive iron accumulation and lipid peroxidation. Excessive mitochondrial fission, due to the overproduction of ROS and increased cellular oxidative levels, is considered a contributing factor to ferroptosis. Ferroptosis in damaged intestines induced by cisplatin was observed alongside increased protein and mRNA expression levels of fission-related proteins Drp1 and Fis1. In contrast, treatment with the antioxidant vitamin D3 inhibited ferroptosis, concurrently reducing ROS accumulation and excessive mitochondrial fission.64 The ferroptosis inducer erastin, in combination with celastrol, mediated cell death in non-small cell lung cancer (NSCLC) cells, accompanied by abundant ROS and enhanced mitochondrial fission.65 Targeting mitochondrial fusion-mediated ferroptosis, increased Mfn1/2-dependent mitochondrial fusion guided by STING1 led to ferroptosis in human pancreatic cancer cells, while knocking down STING1 or Mfn1/2 genes reduced their sensitivity to ferroptosis.66 In a mouse model of brain I/R injury, selenium enhanced Mfn1 expression to promote mitochondrial fusion, significantly reducing oxidative stress and iron accumulation and ultimately ensuring higher mouse survival.67
Regulation of mitochondrial dynamics and disease progression: From energy metabolism to cellular aging
Our findings (Supplementary Fig. 11–15; Table 9, Table 10) indicate that diseases related to mitochondrial dynamics are an important research area. In the case of neurodegenerative diseases, chronic progressive damage to neurons is critical. Given neurons’ high energy metabolic demands and the inability of adenosine triphosphate (ATP) to be internally transported due to rapid hydrolysis, the proper distribution of mitochondria (the sole supplier of ATP) becomes crucial. This distribution depends on altered mitochondrial dynamics and is particularly susceptible to mitochondrial dysfunction, thereby highlighting the importance of mitochondrial dynamics.68, 69, 70
Bibliometric analyses indicate that AD is the most frequently studied neurodegenerative condition in academic literature, with Parkinson’s disease also receiving significant attention. Earlier studies found potential mitochondrial network fragmentation in neuronal brain biopsies from AD patients.71 Subsequent research revealed that amyloid-beta (Aβ) and other AD-related damage directly activate calpains, leading to cleavage of dynamin-like protein 1 (DLP1, a homolog of Drp1) and Mfn2, skewing dynamics towards hyperfission.72 However, it remains controversial that the expression of Drp1 is reduced in AD brains, similar to the fusion proteins (OPA1, Mfn1 and Mfn2), while the levels of the cleavage factor Fis1 are significantly increased.73, 74 Yet, the DLP1 inhibitor Midivi-1 consistently shows early rescue of mitochondrial morphology and motility, as well as alleviation of amyloid pathology and cognitive function improvement, possibly by reducing Aβ production.75, 76 The exact role and mechanism of Drp1 in hyperfission remain to be confirmed.
Parkinson’s disease is the 2nd neurodegenerative disease discussed, also considered a mitochondrial disease, characterized by the gradual death of dopaminergic neurons due to intracellular accumulation of alpha-synuclein (α-syn). Existing evidence supports the localization of α-syn to mitochondria.77, 78 The α-syn influences mitochondrial size by directly acting on the fusion-fission process. In mice overexpressing the A53T α-syn mutant, reduced expression of Mfn1, Mfn2 and Drp1 was observed, with mitochondrial expression of α-syn enhancing the mitochondrial fragmentation phenotype.79 Similarly, in SH-SY5Y cells, overexpression of A53T led to increased translocation and expression of DLP1 targeted to mitochondria.80 Overall, the reliance of neurons on mitochondria determines the irreplaceable importance of mitochondrial dynamics in neurodegenerative diseases, with its application and regulatory roles continually explored.
Moreover, mitochondrial dynamics are involved in the pathogenesis of other diseases. Dysregulation of Opa1 decreased L-Opa1, increased S-Opa1-induced mitochondrial fragmentation and reduced OXPHOS, leading to left ventricular dysfunction and myocardial atrophy.81 Increased mitochondrial fragmentation is a critical factor in reduced OXPHOS, diminishing the ability to oxidize lipids and accumulating undesired lipid species such as triglycerides and diacylglycerol. Notably, increased diacylglycerol promotes protein kinase C (PKC) activation, which disrupts the insulin signaling cascade by inhibiting insulin receptor substrate-1 (IRS-1) activity. This inhibition leads to a reduction in phosphatidylinositol 3-kinase (PI3K) activity, a key factor in insulin signaling. As a result, impaired PI3K signaling contributes to insulin resistance, which is associated with the development of type 2 diabetes.82 In the loss of skeletal muscle mass and strength, exercise training represents a practical therapeutic approach to recovery. Studies confirm that increased expression of Mfn1 and Mfn2 enhances mitochondrial fusion, leading to an enlarged cristae surface area (CSA), which facilitates cross-mitochondrial network junctions. This structural adaptation supports the overall elevated OXPHOS levels by improving mitochondrial efficiency and inter-organelle communication.81, 83 Concurrent pDrp1 Ser616 and Fis1 activity increases are also observed.83, 84 Exercise training-induced increases in Mfn2 and Drp1 expression in skeletal muscles of aging rats salvaged muscle endurance and enhanced exercise performance.85
Limitations
This study also has some limitations. Data were collected using the WoSCC. Although WoSCC strives to minimize bias, this factor in any academic database is complex and multifaceted. The scientific impact of publications, determined by citations, is now commonly regarded as a measure of research quality. However, while citations may assess the scientific influence of a study, they do not necessarily reflect its impact outside the scientific community. Furthermore, numerous factors influence citations, and the meaning of citations can vary widely, including negative citations.86 Much of the literature in WoSCC comes from English-language publishers, which introduces potential language bias.87
Moreover, we added an English-language filter to our search criteria, given its international recognition and evaluation, which may also introduce potential publication and language bias. In future research, we plan to expand the language scope to include a more comprehensive analysis of literature related to mitochondrial dynamics.
Currently, research in mitochondrial dynamics primarily focuses on changes in mitochondrial dynamics in diseases,88 mitochondrial homeostasis and quality control.89 However, significant gaps remain in the upstream regulatory mechanisms of mitochondrial dynamics and the development of drugs targeting mitochondrial dynamics, areas that warrant further investigation by researchers.
Conclusions
Research in mitochondrial dynamics has shown steady and consistent growth. Through bibliometric analysis, we have identified the leading countries contributing significantly to this field, with China and the USA at the forefront, as well as relevant research institutions, authors, journals, and references. Current studies indicate that mitochondrial dynamics are active in a diverse array of mitochondrial-related cellular events, interacting with various forms of PCD and demonstrating potential applications across different diseases. Additionally, mitochondrial dynamics are involved in a wide range of pathophysiological processes and play a crucial role in various diseases such as AD, Parkinson’s disease and diabetes. It highlights their potential as therapeutic targets for disease treatment and underscores the value of developing targeted drugs aimed at mitochondrial dynamics. Our findings open up new research avenues for their potential in disease therapy, which may guide future studies and offer new insights.
Ethics approval and consent to participate
Ethical approval was not applicable because this study is based exclusively on published literature.
Supplementary data
The supplementary materials are available at https://doi.org/10.5281/zenodo.14963828. The package includes the following files:
Supplementary Fig. 1. Visualization of co-cited authors’ network.
Supplementary Fig. 2. Density visualization of co-cited authors.
Supplementary Fig. 3. Journal landscape in mitochondrial dynamics research. Visualizations map the journal landscape within the mitochondrial dynamics research field. Network visualization maps out the 198 journals that have published 332 articles.
Supplementary Fig. 4. Density visualization reveals the concentration of publications across these journals.
Supplementary Fig. 5. Network visualization of co-citation journals.
Supplementary Fig. 6. Density visualization of co-citation journals.
Supplementary Fig. 7. Key references shaping mitochondrial dynamics research. The citation and co-citation networks within mitochondrial dynamics research. Network visualization pinpoints seminal papers with the highest citation counts.
Supplementary Fig. 8. Density visualization highlights the clusters of most frequently cited references.
Supplementary Fig. 9. Network visualization of co-cited references elucidates the interconnectivity between references.
Supplementary Fig. 10. Density visualization of co-cited references further emphasizes the impact and relevance of these works within the scientific community.
Supplementary Fig. 11. Mitochondrial dynamics: A key word analysis of research trends and foci. The critical thematic elements shaping mitochondrial dynamics research. The key word network identifies the most common terms used across 332 articles.
Supplementary Fig. 12. The key word density map provides a visual concentration of these terms, indicating areas of high research activity.
Supplementary Fig. 13. An overlay visualization based on the average publication year shows the evolution of research focus over time, with recent years gravitating toward specific molecular mechanisms.
Supplementary Fig. 14. The timeline view clusters evolving keywords, with the most significant clusters associated with fundamental processes like death and apoptosis. Each horizontal line represents a cluster; the smaller the number, the larger the cluster (No. 0 is the largest). The time is performed at the top, and keywords are located at their first co-occurrence time in the clusters.
Supplementary Fig. 15. Top 25 keywords with the most robust citation bursts (sorted by the starting year). The red bars mean citation burstiness.
Supplementary Fig. 16. Dual-map analysis of mitochondrial dynamics research. The left map shows the citing journals, while the right map represents the cited journals. The paths illustrate the citation flow between disciplines, highlighting the key knowledge sources and research directions in the field.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.

















.jpg)
.jpg)
.jpg)
.png)
.png)
.jpg)
.jpg)
.jpg)
.png)