Abstract
Background. Intracranial aneurysm (IA) is a serious condition that can lead to a life-threatening rupture, often resulting in a hemorrhagic stroke. Vascular smooth muscle cell (VSMC) dysfunction is a critical factor in the pathogenesis of IA, yet the molecular mechanisms underlying this relationship are not yet fully understood. Recent studies suggest that circular RNAs (circRNAs) are involved in various vascular diseases. High-throughput sequencing identified hsa_circ_0008433 as significantly upregulated in IA tissues, especially in ruptured cases, suggesting a role in IA progression.
Objectives. To further investigate the potential effects of hsa_circ_0008433 on the rupture of human IA.
Materials and methods. This study aimed to investigate the effects of hsa_circ_0008433 on IA rupture. We validated the expression of hsa_circ_0008433 in IA patient tissue samples through reverse transcription quantitative polymerase chain reaction (RT-qPCR), comparing ruptured and unruptured aneurysms. Human brain vascular smooth muscle cells (HBVSMCs) were utilized to establish overexpression and knockdown models for hsa_circ_0008433. Cell Counting Kit-8 (CCK-8) and wound healing assays were conducted to assess cell proliferation and migration, while western blotting was employed to measure VSMC phenotype markers including α-smooth muscle actin (α-SMA), smooth muscle protein 22-alpha (SM22α), matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9 (MMP-9).
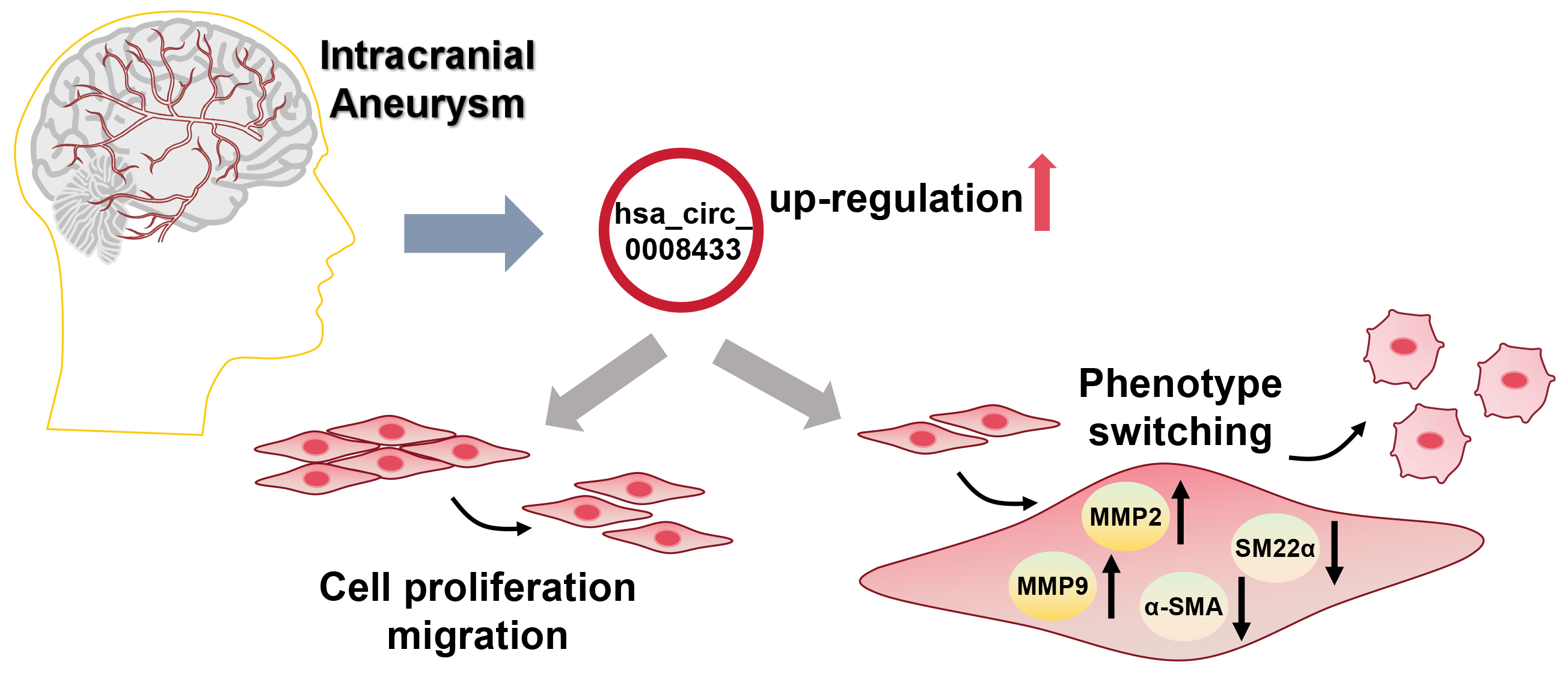
Results. The RT-qPCR analysis confirmed that hsa_circ_0008433 was significantly upregulated in IA tissues, especially in ruptured samples (p < 0.05). Overexpression of hsa_circ_0008433 in HBVSMCs promoted proliferation, migration and phenotype switching, indicated by increased expression of MMPs and decreased contractile proteins. The effects were reversed by the knockdown of hsa_circ_0008433.
Conclusions. We have shown that hsa_circ_0008433 regulates vascular smooth muscle cell function and promotes behaviors that may lead to intracranial aneurysm instability. This study advances the understanding of the role of circRNAs in vascular pathology and identifies hsa_circ_0008433 as a potential therapeutic target for IA. These findings open opportunities for targeted treatments and broader applications in vascular disease research.
Key words: circular RNA, intracranial aneurysm, vascular smooth muscle cell, phenotype, rupture
Background
Intracranial aneurysms (IAs) are arterial swellings within the cerebral vasculature, often resulting from congenital anomalies or acquired injuries, and are a leading cause of aneurysmal subarachnoid hemorrhage.1, 2 Intracranial aneurysm is the 3rd most common cerebrovascular disease after cerebral thrombosis and hypertensive hemorrhage.3 When an IA ruptures, blood enters the subarachnoid space or brain parenchyma, leading to hemorrhagic stroke.4, 5 This abrupt bleeding elevates intracranial pressure, causing cerebral edema, hypoxia and ischemic injury to brain cells.6, 7 The hemorrhage-induced inflammatory response further exacerbates brain by disrupting the blood–brain barrier and triggering secondary injuries, often resulting in severe neurological deficits or death.8, 9 Consequently, IA rupture remains a critical concern in neurosurgical research due to its high fatality rate.10
While IA has been extensively studied, the mechanisms underlying its formation and rupture are not fully understood. Emerging research highlights the crucial role of vascular smooth muscle cells (VSMCs) in IA pathophysiology.11, 12 Under healthy conditions, VSMCs maintain a contractile phenotype, supporting vascular stability. However, in pathological states, VSMCs can switch from a contractile to a pro-inflammatory phenotype.13, 14 This phenotypic switch involves decreased expression of contractile proteins, such as α-smooth muscle actin (α-SMA), and increased expression of matrix metalloproteinases (MMPs) that degrade the extracellular matrix (ECM).15, 16 These transformations can destabilize the vascular structure, heightening the risk of IA rupture. The phenotypic diversity of VSMCs is therefore central to the formation, progression and rupture of IAs.17, 18
Circular RNA (circRNA)s represents a novel class of non-coding RNA characterized by a stable closed-loop structure, which makes it highly stable compared to linear RNA.19, 20 Unlike linear RNAs, circRNA lacks a 5’ cap and 3’ poly(A) tail, making it resistant to exonucleases and thus more stable.21, 22 Recent studies show that circRNAs can act as “sponges” for microRNAs (miRNAs) and modulate target gene expression through a competing endogenous RNA (ceRNA) mechanism.23, 24 Circular RNAs have been implicated in several diseases, including diabetes, neurological disorders, cardiovascular disease (CVD), and cancer.25, 26, 27, 28 Within the cerebrovascular system, circRNAs have been associated with key processes such as angiogenesis, autophagy, apoptosis, and inflammation.29, 30 They are also believed to regulate VSMC phenotype and might even act as miRNA carriers within IAs.31, 32 Recent studies indicate that circRNAs are essential in regulating the phenotype of human brain vascular smooth muscle cells (HBVSMCs).33 Changes in circRNA expression may influence IA formation and rupture by modulating the proliferation, migration and phenotype switching of HBVSMCs. Although circRNAs hold significant potential in vascular diseases, their specific role in IA formation and rupture has not been thoroughly studied.27 Furthermore, the relationship between circRNA-regulated HBV-SMC proliferation and phenotype switching, as well as its role in the overall pathophysiology of IA, remains unclear. This gap provides a rationale and direction for this study.
In our previous research, high-throughput sequencing identified hsa_circ_0008433 as significantly upregulated in human IA tissues, especially in ruptured aneurysms.34, 35, 36, 37 This finding suggests that hsa_circ_0008433 may play an important role in IA pathology, particularly in the critical and life-threatening event of aneurysm rupture. Based on these preliminary findings, we hypothesize that hsa_circ_0008433 may influence VSMC behavior under pathological conditions, potentially promoting VSMC proliferation, migration and phenotype switching, thereby affecting IA stability and ultimately contributing to aneurysm rupture.
Objectives
This study aims to further investigate the role of hsa_circ_0008433 in HBVSMCs, specifically in promoting cell proliferation, migration and phenotype switching. By gaining a deeper understanding of the molecular mechanisms underlying hsa_circ_0008433’s involvement in IA pathology, we hope to provide new insights for future therapeutic strategies, supporting clinical interventions for cerebrovascular diseases.
Materials and methods
Study design
By analyzing tissue samples from IA patients and healthy volunteers, combined with in vitro transfection experiments, we compared the expression levels of hsa_circ_0008433 and its effects on HBVSMC proliferation, migration and phenotype switching.32
Participants
Healthy volunteers (n = 15) and patients with IA (n = 15) who were admitted to our Department of Neurosurgery of the Second Affiliated Hospital of Fujian Medical University (Quanzhou, China) in 2019 were included in the analysis. Tissues from the aneurysm wall of IA patients were considered the IA wall group, while tissues from the superficial temporal artery of healthy volunteers served as the control group. Additionally, the IA tissues were categorized into 2 subgroups based on the aneurysmal state: the ruptured group (n = 7) and the unruptured group (n = 8). The study was approved by the Ethics Committee of The Second Affiliated Hospital of Fujian Medical University (approval No. 2021-434).
Cell cultivation and group divide
For in vitro experiments, HBVSMCs (cat. No. PCS-100-012) were purchased from the American Type Culture Collection (ATCC; Manassas, USA) and cultured in a special complete medium (CM-H116; Procell, Wuhan, China) at 37°C and 5% CO2. Human brain vascular smooth muscle cells transfected with negative siRNA and hsa_circ_0008433 siRNA were designated as the si-NC group and the si-hsa_circ_0008433 group, respectively. Besides, pLC5-circ plasmid was used to transfect HBVSMCs to over-express hsa_circ_0008433. The normal HBVSMCs without any treatment and HBVSMCs transferred with pLC5 circ plasmid were named the blank group and the vector group, respectively. Both pLC5 circ plasmid and siRNA were designed and purchased from Geneseed Biotech Co., Ltd. Cell transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) according to themanufacturer’s instructions.
Quantitative reverse transcription polymerase chain reaction
The HBVSMCs were split using TRIzol (Thermo Fisher Scientific, Waltham, USA) and total RNA was separated according to themanufacturer’s instructions. Then, 1 μg RNA was reverse transcribed using reverse transcriptase kits of miRNA or M-MLV (Moloney murine leukemia virus reverse transcriptase; Thermo Fisher Scientific, again following the manufacturer’sinstructions. QuantStudio5™ Real-Time PCR System (Applied Biosystems, Foster City, USA) was adopted for a quantitative reverse transcription polymerase chain reaction (RT-qPCR). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the internal control and a 2–ΔΔCt method was used to measure relative expression. The sequence results for circRNA were derived from circBase (http://circrna.org). The primer pairs were synthesized by Sangon Biotech (Shanghai, China).
Cell Counting Kit-8
After the above grouping and treatment, HBVSMCs were seeded into 96 well plates at a density of 5 × 104 cells/well at 37°C overnight. Subsequently, 10 μL Cell Counting Kit-8 (CCK-8) solution (Abcam, Cambridge, USA) was added to each well for 4 h of incubation at 37°C. Then, the absorbance at 450 nm was measured using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, USA). Each sample was tested independently in triplicate.38
Wound healing assay
Cell migration was observed using wound healing assay. Cells (2 × 105) were seeded in a 6-well plate after the above-mentioned grouping and arrangement. When the cells reached 90% confluence, a sterile micropipette tip was used to scratch the cell monolayer, forming the wound. Simultaneously, the cells were cultured in a serum-free medium. After 24 h, the wound area was examined using a light microscope (CKX41; Olympus Corp., Tokyo, Japan). Additionally, ImageJ software v. 1.41 (National Institutes of Health (NIH), Bethesda, USA) was used to measure the wound width. Finally, cell migration capacity was expressed as a percentage of original wound distance.
Western blotting
Cells were lysed using radio immunoprecipitation assay buffer (RIPA; Beyotime, Shanghai, China), followed by centrifugation at 10,000 × g for 5 min. Protein concentrations were determined using a bicinchoninic acid (BCA) kit (Thermo Fisher Scientific). Then, 25 µg of protein per sample was loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) for electrophoresis. Following separation, the proteins were transferred onto a polivinylidene difluoride (PVDF) membrane. The membrane was subsequently blocked with 5% skimmed milk powder (Solarbio, Beijing, China) for 2 h to prevent nonspecific binding. It was then incubated overnight at 4°C with the following primary antibodies: anti-α-SMA (ab7817, 1:10,000; Abcam), anti-smooth muscle protein 22-α (SM22α) (ab1410 6, 1:10,000; Abcam), anti-MMP-9 (ab76003, 1:500; Abcam), anti-MMP-2 (ab92536, 1:1,000; Abcam), and anti-GAPDH (ab37168, 1:10,000; Abcam). Next, the membrane was washed and incubated with horseradish peroxidase (HRP)-linked secondary antibody (Abcam) at ambient temperature for 2 h. Protein bands were then analyzed using the BeyoECL Plus kit (Solarbio) and quantified using ImageJ. For blot analysis, GAPDH was used as a standardized reference.
Statistical analyses
Statistical analysis was conducted using IBM SPSS v. 26.0 (IBM Corp., Armonk, USA). Data are presented as median (interquartile range (IQR)). The Mann–Whitney U test was used to compare significant differences between the 2 groups. For comparisons involving 3 or more groups, the Kruskal–Wallis test was applied, followed by Dunn’s post hoc test for multiple comparisons. Statistical significance was set at p < 0.05.
Results
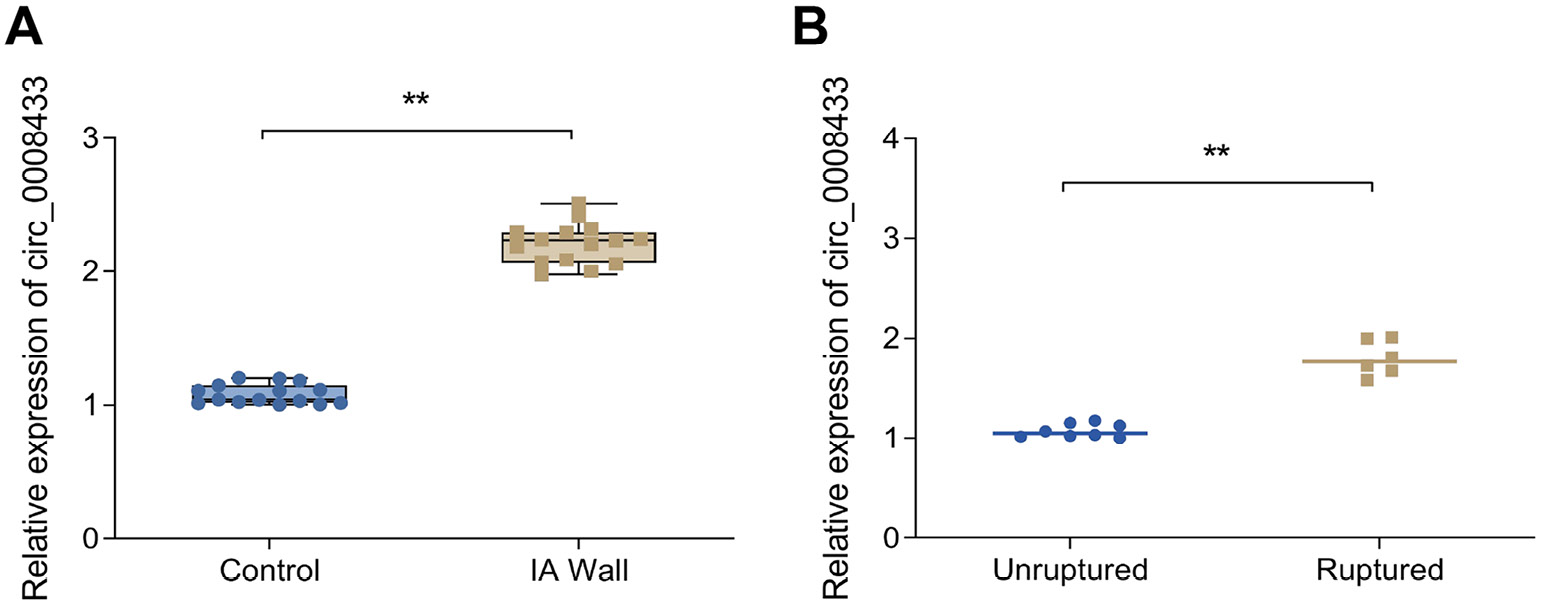
Upregulation of hsa_circ_0008433 in intracranial aneurysm tissues and intracranial aneurysm rupture tissues
To confirm the expression of hsa_circ_0008433, we performed RT-qPCR on aneurysm wall samples. As shown in Figure 1A, Table 1 and Supplementary Table 1, hsa_circ_0008433 expression levels were notably higher in the IA wall group compared to the control group. Additionally, Figure 1B and Table 2 show that hsa_circ_0008433 expression was increased in the ruptured group compared to the unruptured group. These findings suggest a close association between high hsa_circ_0008433 expression and IA pathogenesis.
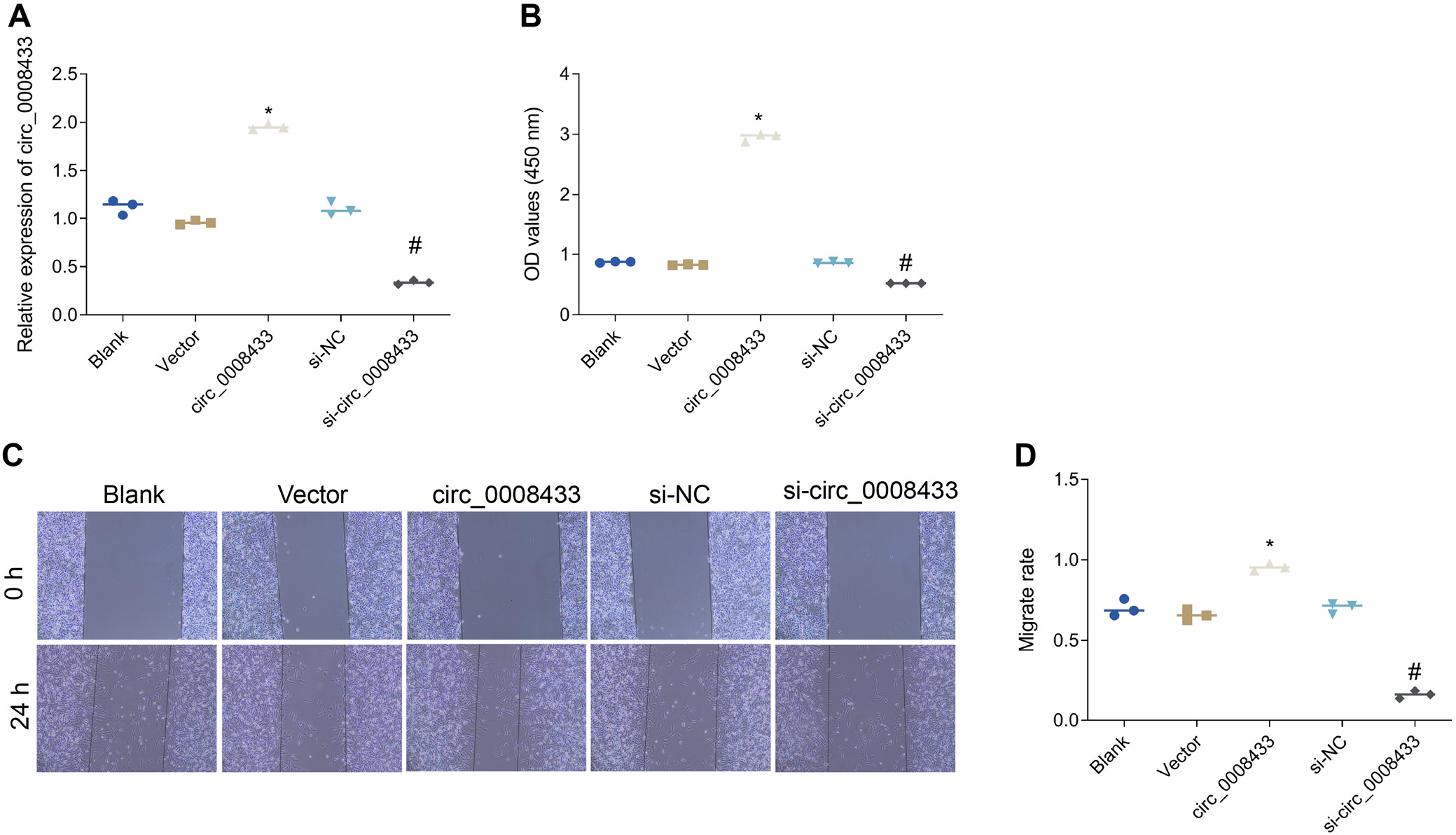
Overexpression of hsa_circ_0008433 promotes the proliferation and migration of HBVSMCs
Overexpression of hsa_circ_0008433 significantly upregulated its expression levels and enhanced cell viability, indicating a promote effect on cell proliferation (Figure 2, Table 3, Table 4, Table 5 and Supplementary Tables 2–4). Conversely, knocking down hsa_circ_0008433 reduced its expression levels and decreased cell viability, underscoring its essential role in cell survival. Wound healing assays further demonstrated that hsa_circ_0008433 overexpression significantly enhanced HBVSMCs’ migratory capacity, crucial for vascular repair, while knockdown reduced migration rates. Overall, these findings indicate that hsa_circ_0008433 promotes HBVSMC proliferation and migration, highlighting its potential importance in vascular repair and remodeling mechanisms.
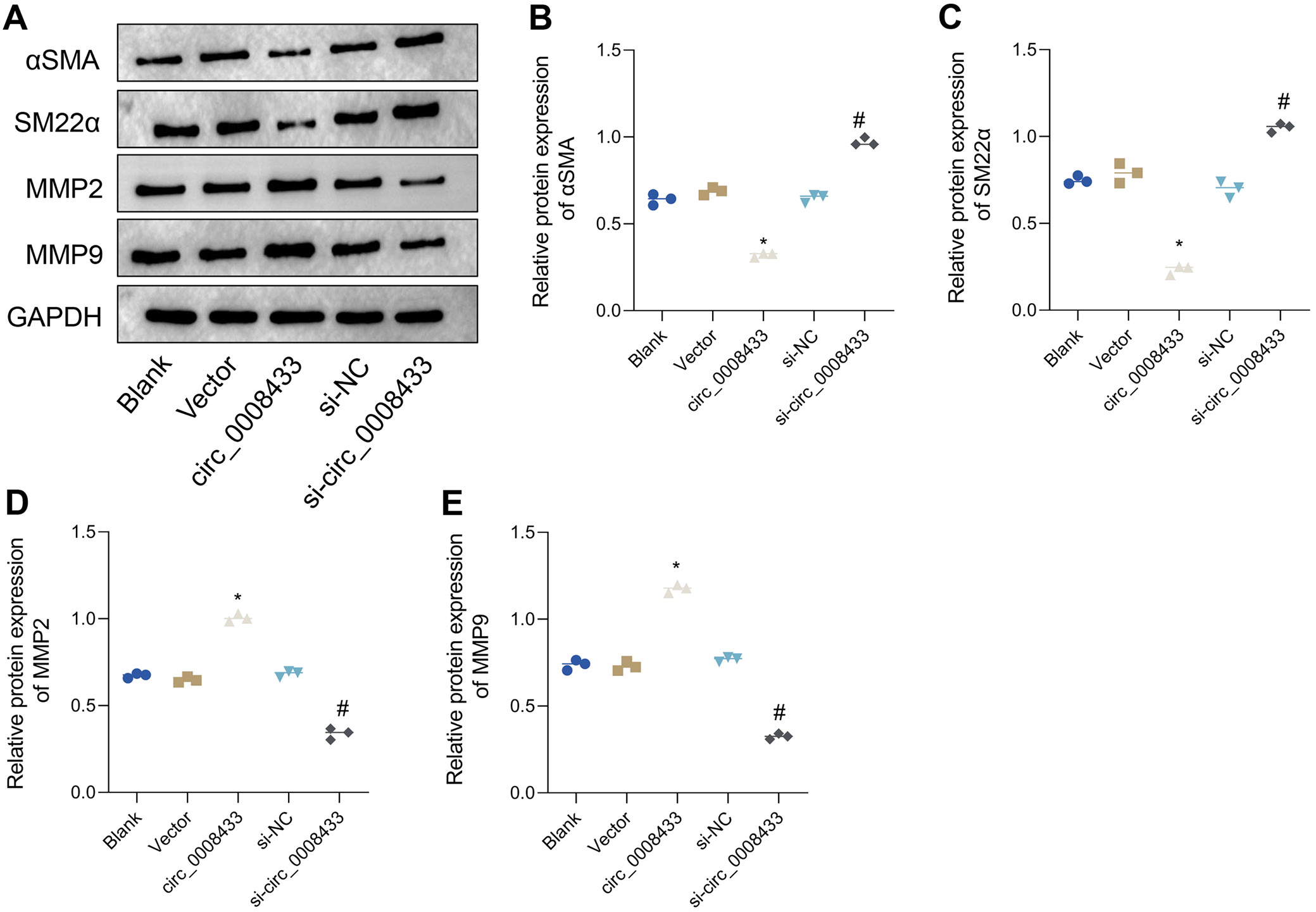
Overexpression of hsa_circ_0008433 promotes phenotype switching of HBVSMCs
To investigate the effect of hsa_circ_0008433 on the phenotype switching of HBVSMCs, we assessed the expression levels of key proteins associated with this process. There were no significant differences in α-SMA, SM22α, MMP-2, and MMP-9 expression levels among the blank, vector and si-NC groups. However, the overexpression of hsa_circ_0008433 led to a significant decrease in α-SMA and SM22α levels and an increase in MMP-2 and MMP-9 levels, as compared to the vector group (Figure 3A–C, Table 6 and Supplementary Tables 5–7). Conversely, knockdown of hsa_circ_0008433 resulted in increased α-SMA and SM22α levels and decreased MMP-2 and MMP-9 levels, compared to the si-NC group (Figure 3A,D,E and Supplementary Tables 8–11). These findings suggest that hsa_circ_0008433 overexpression promotes phenotype switching in HBVSMCs, favoring a shift towards a pro-inflammatory state.
Discussion
This study provides a comprehensive investigation into the role of hsa_circ_0008433 in IA pathogenesis, a condition characterized by high morbidity and mortality due to its potential for rupture, which can lead to life-threatening subarachnoid hemorrhage.39, 40 Prior high-throughput sequencing indicated that hsa_circ_0008433 is significantly upregulated in IA tissues, especially in ruptured cases, suggesting its involvement in IA pathology.27 Our study builds on these findings by verifying the overexpression of hsa_circ_0008433 through RT-qPCR, particularly in aneurysm wall tissues, confirming its close association with IA. Through in vitro experiments, we reveal the functional role of hsa_circ_0008433 in promoting HBVSMC proliferation, migration and phenotype switching, highlighting its potential as a key factor in IA development and instability. There is an increasing body of literature indicating that various circRNAs are involved in regulating HBVSMCs in IA and other cerebrovascular diseases,41 underscoring the relevance of our findings. For example, hsa_circ_002039 has been shown to be downregulated in IA patient tissues and to promote VSMC proliferation.42, 43 Similarly, hsa_circ_0021001 is regarded as a potential biomarker for IA, with low expression levels correlating with poorer clinical outcomes.44 Another circRNA, hsa_circ_0031608, is highly expressed in pro-inflammatory VSMCs and aortic dissection, where it promotes HBVSMC migration and proliferation, contributing to vascular remodeling.45 However, these studies often do not delve into the precise mechanisms of action for these circRNAs. By contrast, our study provides a systematic analysis of hsa_circ_0008433, demonstrating not only its expression in IA tissues but also its functional impact on HBVSMCs, thus shedding light on its potential mechanisms of action.
Our in vitro experiments show that knocking down hsa_circ_0008433 significantly reduces HBVSMC proliferation and migration, emphasizing its essential role in maintaining these functions under pathological conditions. Previous studies have highlighted the importance of VSMC phenotype switching in the formation and rupture of IAs.46 Under normal physiological conditions, VSMCs adopt a contractile phenotype that supports vascular stability. However, in response to pathological stimuli, VSMCs can shift to a pro-inflammatory or matrix-remodeling phenotype, characterized by an upregulation of MMPs (MMP-2 and MMP-9) and a downregulation of contractile proteins such as α-SMA and SM22α.15, 47, 48, 49, 50, 51 This switch is associated with increased proliferative and migratory capacities, which are more commonly observed in ruptured IA tissues than in unruptured ones,51, 52 suggesting a link to aneurysm wall remodeling and rupture. Our findings indicate that hsa_circ_0008433 may be instrumental in facilitating this phenotype switching. Specifically, silencing hsa_circ_0008433 led to reduced MMP-2 and MMP-9 expression and increased α-SMA and SM22α levels, suggesting that hsa_circ_0008433 drives HBVSMCs towards a pro-inflammatory phenotype that compromises aneurysm wall stability.
These findings imply that hsa_circ_0008433 plays a modulatory role in vascular cell behavior within the aneurysmal environment, providing new insights into IA pathophysiology. Understanding hsa_circ_0008433’s influence on HBVSMCs facilitates novel therapeutic strategies. If hsa_circ_0008433 indeed promotes HBVSMC proliferation, migration and phenotype switching, then targeting this circRNA could help stabilize aneurysmal walls and potentially prevent rupture. Additionally, given its upregulation in IA tissues, hsa_circ_0008433 may serve as a diagnostic or prognostic biomarker, aiding in early detection and risk assessment in high-risk IA patients.
Looking ahead, future research should focus on several key areas. First, validating these findings in vivo would provide a clearer picture of the role of hsa_circ_0008433 in IA stability and rupture risk. Developing animal models that can manipulate hsa_circ_0008433 levels would help determine whether modifying its expression could reduce aneurysm rupture risk or prevent aneurysm formation altogether. Second, a deeper exploration of the molecular pathways associated with hsa_circ_0008433’s effects on HBVSMC behavior is necessary. By investigating its interactions with pathways such as mitogen-activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and transforming growth factor beta (TGF-β), we could better understand how this circRNA influences HBVSMC function and IA pathophysiology. Third, hsa_circ_0008433’s potential as a therapeutic target warrants further exploration. If effective delivery systems can be developed to modulate hsa_circ_0008433 levels or inhibit its function in HBVSMCs, it may be possible to stabilize aneurysmal walls in high-risk IA patients, offering a non-surgical alternative for those unable to undergo traditional interventions.
This study underscores the importance of translational research by linking hsa_circ_0008433 mechanisms in HBVSMCs to potential clinical applications, thereby accelerating lab-to-patient translation.53 Additionally, exploring neural mechanisms in IAs may clarify cognitive impairments in patients, as studies indicate reduced local gyrification, cognitive deficits and white matter lesions in unruptured IA cases.54, 55, 56 Integrating neuroimaging data with circRNA research could deepen our understanding of IA’s impact on brain function.
Limitations
While our findings are promising, this study has several limitations. First, the relatively small sample size of clinical IA tissues may limit the generalizability of our results. Larger studies across multiple centers are necessary to confirm these findings and to validate the robustness of hsa_circ_0008433 as a biomarker.16 Second, this research is limited to in vitro experiments, which, while valuable for understanding molecular mechanisms, do not fully capture the complex in vivo environment of the human brain. The pathology of IA in living organisms involves a multitude of factors – including hemodynamic forces, inflammatory mediators and immune cells – that are absent in cell culture models. Therefore, animal models or clinical studies would be essential for confirming the pathophysiological relevance of hsa_circ_0008433 in IA.17 Third, this study focuses exclusively on a single circRNA, hsa_circ_0008433, without considering potential interactions with other circRNAs that may contribute to IA pathogenesis. Future studies should adopt a more comprehensive approach, examining multiple circRNAs to develop a fuller understanding of the regulatory networks at play in IA.18, 19 Other confounding factors may also influence hsa_circ_0008433 expression and IA risk, such as patient age, gender and pre-existing conditions like hypertension or diabetes. Future research should consider these variables in study design and analysis to better understand how they may modulate circRNA expression and impact IA pathology. Additionally, while this study demonstrated the impact of hsa_circ_0008433 on MMP-2, MMP-9, α-SMA, and SM22α levels, the underlying molecular pathways remain unclear. It is essential to explore potential interactions with major signaling pathways, such as MAPK, NF-κB and TGF-β, which are known to play roles in vascular inflammation and remodeling.57, 58, 59 Understanding these interactions could reveal the precise mechanisms by which hsa_circ_0008433 regulates HBVSMC phenotype and IA formation.
Conclusions
Overall, our study demonstrates that hsa_circ_0008433 plays a crucial role in the proliferation and migration of VSMCs in IA patients and may be involved in the phenotype modulation of HBVSMCs. These findings suggest that hsa_circ_0008433 is associated with the pathological processes of IAs. The upregulation of hsa_circ_0008433 in IA tissues indicates its potential as a biomarker for the diagnosis and prognosis of IAs. Clinically, targeting hsa_circ_0008433 could lead to novel therapeutic strategies to prevent the progression or rupture of IAs. Future research should aim to elucidate the detailed molecular mechanisms by which hsa_circ_0008433 regulates HBVSMCs behavior, confirm these findings in vivo, and explore the broader network of circRNAs involved in cerebrovascular diseases. These efforts will contribute to a more comprehensive understanding of IA pathophysiology and support the development of targeted therapies to improve patient outcomes.
Supplementary data
The supplementary materials are available at https://doi.org/10.5281/zenodo.14997708. The package includes the following files:
Supplementary Table 1. Checking the assumption of normality (using the Shapiro–Wilk test).
Supplementary Table 2. Dunn’s post hoc test results for group comparisons of circ_0008433 expression in Figure 2A.
Supplementary Table 3 Dunn’s post hoc comparison of cell viability between groups in Figure 2B.
Supplementary Table 4. Dunn’s post hoc test results, pairwise comparison of migration capacity between groups in Figure 2D.
Supplementary Table 5. Dunn’s post hoc test results, pairwise comparison of α-SMA protein expression levels between groups in Figure 3B.
Supplementary Table 6. In the analysis comparing the relative expression levels of SM22α protein among groups (Figure 3C), the Kruskal–Wallis test was used to evaluate the differences between the groups. The results showed an H value of 12.23 and a p-value of 0.016, indicating significant differences between the groups.
Supplementary Table 7. Dunn’s post hoc test results, pairwise comparison of SM22α protein expression levels between groups in Figure 3C.
Supplementary Table 8. In the analysis comparing the relative expression levels of MMP-2 protein among groups (Figure 3D), the study used the Kruskal–Wallis test to evaluate the differences between the groups.
Supplementary Table 9. Dunn’s post hoc test results, pairwise comparison of MMP-2 protein expression levels between groups in Figure 3D.
Supplementary Table 10. In the analysis comparing the relative expression levels of MMP-9 protein among groups (Figure 3E), the Kruskal–Wallis test was used to evaluate the differences between the groups.
Supplementary Table 11. Dunn’s post hoc test results, pairwise comparison of MMP-9 protein expression levels between groups in Figure 3E.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.