Abstract
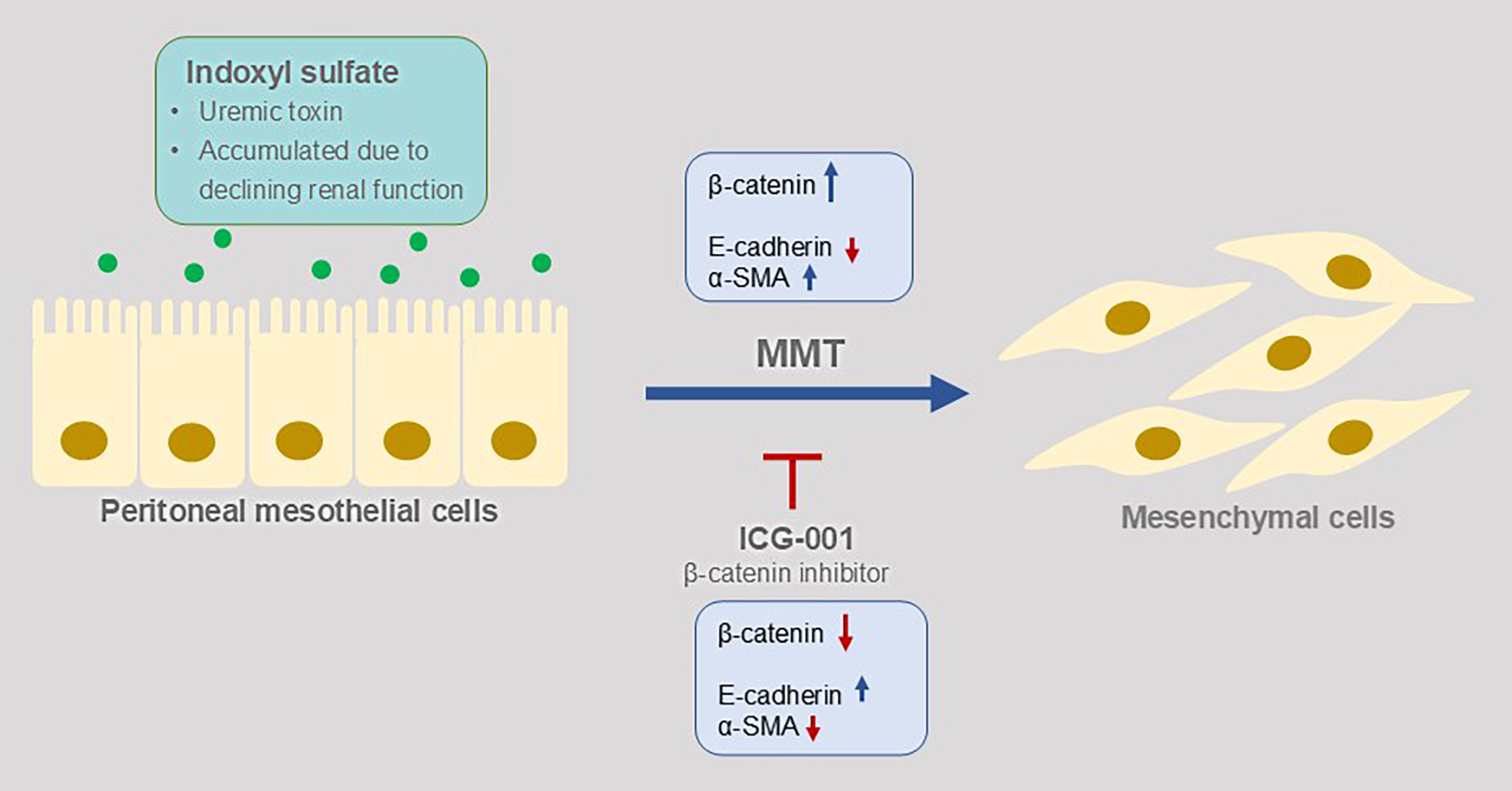
Background. Long-term peritoneal dialysis (PD) leads to peritoneal injury, with mesothelial-to-mesenchymal transition (MMT) potentially serving as an initial and reversible stage of this process. Indoxyl sulfate (IS), a protein-bound uremic toxin that accumulates in patients with declining renal function, is known to be associated with epithelial–mesenchymal transition (EMT) in proximal renal tubular cells. However, its effects on peritoneal mesothelial cells, which serve as the first-line barrier during PD, have not yet been investigated.
Objectives. This study aimed to evaluate whether IS induces MMT in human peritoneal mesothelial cells during PD through the β-catenin signaling pathway.
Materials and methods. A human peritoneal mesothelial cell line (HMrSV5) was used for this in vitro study. Cells were treated with IS or combined with β-catenin inhibitor ICG-001, and high glucose PD fluid (PDF) served as a positive control. Morphology, proliferation and adhesion were assessed, while the expression of β-catenin and α-smooth muscle actin (α-SMA) as mesenchymal markers, along with E-cadherin as a mesothelial marker, were analyzed at both RNA and protein levels using real-time polymerase chain reaction (PCR) and western blot, respectively.
Results. The number of viable and adherent cells was significantly increased in the IS and PDF groups compared to the control (p < 0.05). Treatment with ICG-001 significantly reduced both viable and adherent cell numbers compared to cells treated with IS or PDF alone (p < 0.05). At the RNA level, IS treatment significantly decreased E-cadherin expression (p = 0.002) while significantly increasing β-catenin (p = 0.001) and α-SMA (p = 0.002) expression compared to the control group. These changes were reversed by ICG-001 treatment. Protein expression showed similar trends.
Conclusions. Indoxyl sulfate induces MMT in human peritoneal mesothelial cells, and these changes can be reversed by the specific β-catenin inhibitor ICG-001. This suggests that IS may be considered as another inducer of MMT during PD through the β-catenin signaling pathway.
Key words: indoxyl sulfate, β-catenin, epithelial–mesenchymal transition, peritoneal dialysis, mesothelial-to-mesenchymal transition
Introduction
Peritoneal dialysis (PD) is one of the renal replacement therapies (RRTs) administered to patients with end-stage renal disease (ESRD). Peritoneal dialysis uses the peritoneum as a living dialysis membrane to clear waste and extra fluid. Compared to hemodialysis (HD), PD offers a lower risk of infection, greater patient mobility and an improved quality of life. Additionally, PD accounts for more than 10% of RRTs worldwide.1, 2, 3 However, long-term PD leads to peritoneal injury, resulting in structural and functional deterioration of the peritoneum, including peritoneal fibrosis and a progressive loss of ultrafiltration. These complications are the primary reasons for PD discontinuation.4, 5, 6 Furthermore, peritoneal fibrosis has been associated with increased morbidity and mortality in patients who have undergone PD.7, 8, 9
Peritoneal fibrosis is a progressive and complex pathological process involving multiple cell types, signaling pathways and molecular interactions. In response to inflammatory or profibrotic stimuli, peritoneal and immune cell populations expand, triggering specific signaling pathways that drive inflammation and ultimately lead to fibrosis. Among these intricate processes, mesothelial cells play a central role in peritoneal membrane alterations through mesothelial-to-mesenchymal transition (MMT), a key mechanism contributing to fibrosis.³
Mesothelial-to-mesenchymal transition is a special type of epithelial–mesenchymal transition (EMT) that occurs in mesothelial cells; it is a process in which epithelial cells lose polarity and adhesion and acquire mesenchymal features, including infiltration and migration ability, accompanied by downregulated epithelial markers such as E-cadherin, and upregulated mesenchymal markers such as α-smooth muscle actin (α-SMA).10 The EMT has been known as the initial change of fibrosis that starting by transforming growth factor beta (TGF-β)-induced signaling pathway in several organs, including the heart, liver and kidney.11, 12, 13 In addition, MMT in peritoneal mesothelial cells has been observed in peritoneal injury cell model using primary and immortal peritoneal mesothelial cell lines in vitro, chronic kidney disease (CKD) and peritoneal injury animal models in vivo, and clinically in PD patients.14, 15, 16
The definite inducer of MMT and sequential peritoneal injury in PD patients is still uncertain. Currently, long-term PD fluid (PDF) exposure has mainly been considered to cause peritoneal injury in PD patients because of its hyperosmotic, hyperglycemic and acidic features.4, 15 In addition to PDF, we hypothesized that long-term exposure of uremic toxins may be also one cause of MMT of peritoneal mesothelial cells based on previous studies. Indoxyl sulfate (IS) is one of the protein-bound uremic toxins that accumulate in patients with declining renal function because of impaired clearance from the circulation. The removal of protein-bound uremic toxins is more challenging than that of other nitrogenous wastes due to their strong affinity for proteins, regardless of whether elimination occurs via HD or PD.17, 18 In addition to a sign of declining renal function, mounting evidence demonstrates that IS has a direct pathogenic impact on proximal tubular cells, leading to tubular atrophy and renal fibrosis.19 Indoxyl sulfate is shown to be associated with EMT and induces the expression of pro-fibrotic markers such as TGF-β, fibronectin and α-SMA in proximal renal tubular cells.20 Furthermore, downregulated E-cadherin and upregulated α-SMA were observed in renal tissue in a CKD mouse model, and the fibrotic area was suppressed in mice treated with AST-120, which inhibits the absorption of indole, the IS precursor, indicating the possible role of IS in the process of EMT and renal fibrosis.21 Evidence demonstrates that EMT combined with chronic inflammation and oxidative stress caused by IS may lead to CKD and progress further to renal fibrosis. In addition, the IS level may serve as a prognostic predictor for CKD, given that the relationship between CKD complications and poor patient outcomes has been demonstrated.22, 23, 24
The possibility of the abovementioned alteration may also exist in the case of MMT in peritoneal mesothelial cells of patients who underwent long-term exposure of IS due to PD. The EMT is an early and reversible process in the development of organ fibrosis, including kidney fibrosis.25 Since fibrosis and subsequent organ failure currently lack effective treatments, developing interventions that target critical signaling molecules involved in EMT, based on our understanding of potential inducers and underlying mechanisms, could help treat or prevent fibrosis. Such approaches may prolong PD technique survival and improve outcomes for patients.
Objectives
The present study evaluated whether IS induced MMT in peritoneal mesothelial cell line (HMrSV5). Since TGF-β, Wnt and Notch extracellular domain (NECD) signaling pathways play important roles in the fibrosis process,25 β-catenin as the common molecule of both TGF-β and Wnt signaling pathways was observed to determine the possible molecular mechanism of IS-induced MMT in peritoneal mesothelial cells.
Materials and methods
Cell culture and treatment
A human peritoneal mesothelial cell line (HMrSV5) was purchased from Shanghai Guandao Bio Co., Ltd (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Thermo Fisher Scientific, Waltham, USA) with 10% fetal bovine serum (FBS; Gibco) at 37°C with 5% CO2 until 80% confluence. Cells were transferred to serum-free DMEM medium overnight before treatment. Cells in the control group were maintained in serum-free DMEM. Cells in the IS and IS+ICG-001 groups were treated with 1 mmol/L IS (Sigma-Aldrich, Shanghai, China) with or without 5 μmol/L ICG-001 (Meilunbio, Dalian, China), respectively, for 12, 24, 48, or 72 h. Cells in the PDF and PDF+ICG-001 groups were treated with PD fluid with 4.25% dextrose (Baxter Healthcare, Shanghai, China) with or without 5 μmol/L ICG-001, respectively, for 12, 24, 48, or 72 h. The PDF served as a positive control. The concentrations and treatment periods of IS and ICG-001 were chosen according to the results of our preliminary test based on previous studies.26, 27 Cell morphology was observed under an inverted phase-contrast microscope (model BX43; Olympus Corp., Tokyo, Japan).
Cell proliferation assay
Cells were seeded (2 × 103 per well) into a 96-well plate and cultured in serum-free DMEM medium overnight before treatment. After treatment, the proliferation of treated cells in each group was assessed using Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan) according to the manufacturer’s instruction. Absorbance was measured at 450 nm.
Adhesion assay
Cells were treated as described above for 48 h and then resuspended in serum-free RPMI-1640 (Hyclone, Logan, USA) to 2 × 104 per well and plated in Matrigel® (BD Biosciences, San Jose, USA)-coated 96-well plates at 37°C for 1 h. Non-adherent cells were removed by washing the plates with phosphate-buffered saline (PBS). Adherent cells were fixed with 4% phosphate-buffered paraformaldehyde for 30 min, stained with 0.5% crystal violet for 15 min and counted under an inverted phase-contrast microscope (model BX41; Olympus Corp.).
RNA extraction and RT-PCR
The isolation of RNA was carried out using the TRIzol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. The preparation of cDNA was accomplished by employing the Prime-Script RT Master Mix (TaKaRa, Kusatsu, Japan). Transcript levels were quantified on the 7900 HT Real-Time PCR System (Applied Biosystems, Foster City, USA) using SYBR Premix Ex Taq (TaKaRa). Relative gene expression was determined using the 2–ΔΔCt method and normalized to the housekeeping gene GAPDH. Primers used were as follows:
E-cadherin, forward: GCCGAGAGCTACACGTTCAC,
reverse: GTCGAGGGAAAAATAGGCTG;
β-catenin, forward: AAAGCGGCTGTTAGTCACTGG,
reverse: CGAGTCATTGCATACTGTCCAT;
α-SMA, forward: CTATGAGGGCTATGCCTTGC,
reverse: GCTCAGCAGTAGTAACGAAGGA;
GAPDH, forward: GGAGCGAGATCCCTCCAAAAT,
reverse: GGCTGTTGTCATACTTCTCATGG.
Western blot
Treated cells were harvested and lysed with phenylmethylsulfonyl fluorid (PMSF)-contained radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China) on ice for 30 min. Lysed cells were centrifuged at 4°C for 20 min, supernatants were collected. Protein was quantified using the Bradford method, equal amount of protein of each group was separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to 0.2 μm polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, USA). The membrane was blocked in 5% dried skimmed milk powder in tris-buffered saline with Tween (TBST) solution at room temperature for 2 h and probed with primary antibodies against E-cadherin (1:1000; Cell Signalling Technology (CST), Danvers, USA), α-SMA (1:1000; Abcam; Cambridge, USA), β-catenin (1:5000; Abcam), or GAPDH (1:1000; Abcam) overnight at 4°C, followed by incubated in appropriate secondary antibodies conjugated with horseradish peroxidase (HRP; 1:2,000; ABclonal, Wuhan, China) at room temperature for 1 h. Protein bands were visualized by electrochemiluminescence (ECL) western blotting detection system (GE Healthcare Life Sciences, Logan, USA) and the intensity was measured using Image Pro Plus v. 6.0 software (Media Cybernetics, Rockville, USA).
Statistical analyses
All data were entered into IBM SPSS v. 22.0 statistical software (IBM Corp., Armonk, USA) for statistical analysis, while GraphPad Prism 6 (GraphPad Software, San Diego, USA) and Adobe Photoshop (Adobe Inc., Mountain View, USA) were used for chart making. The Shapiro–Wilk test was used to assess the normality of the distribution for the data. Due to the non-normal distribution of data, the Kruskal–Wallis test was used for group comparisons, followed by Dunn’s post hoc test. A p-value <0.05 was considered statistically significant. Results were presented as box-and-whisker plots, with all data points displayed as individual dots. The sample size for each experiment ranged from 3 to 4, as indicated in the corresponding figure legends.
Results
MMT-related cell characteristics
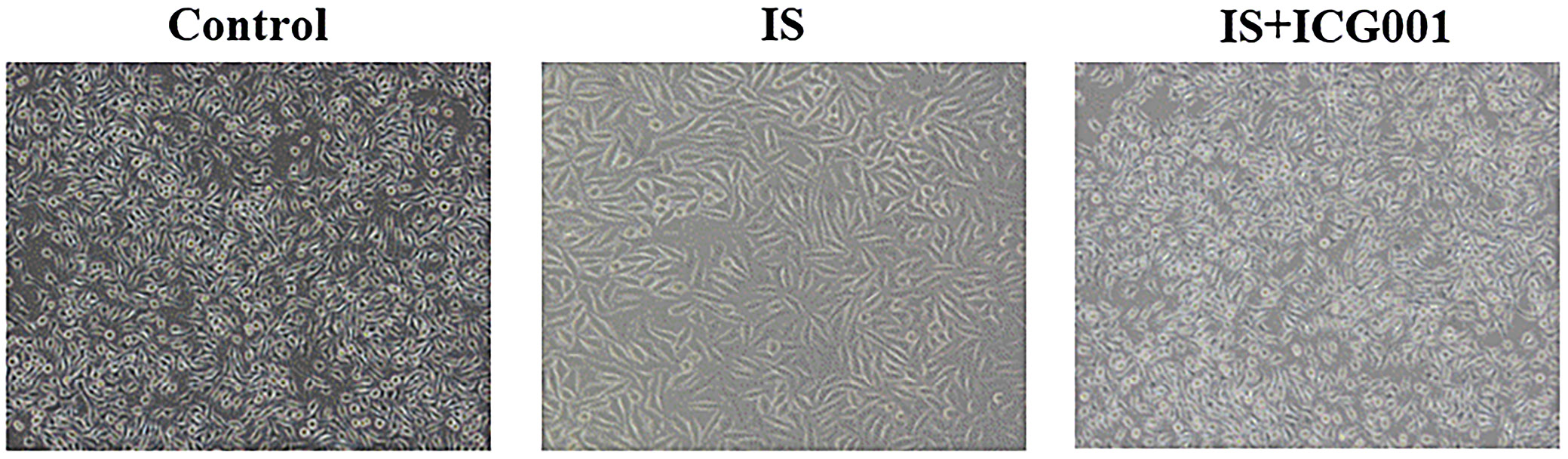
Indoxyl sulfate induced morphological changes in HMrSV5 cells, shifting from the rounded appearance observed in the control group to an elongated, fibroblast-like morphology. These changes were mitigated by the addition of the β-catenin inhibitor ICG-001, as evidenced by the cuboidal shape of cells in the IS+ICG-001 group (Figure 1).
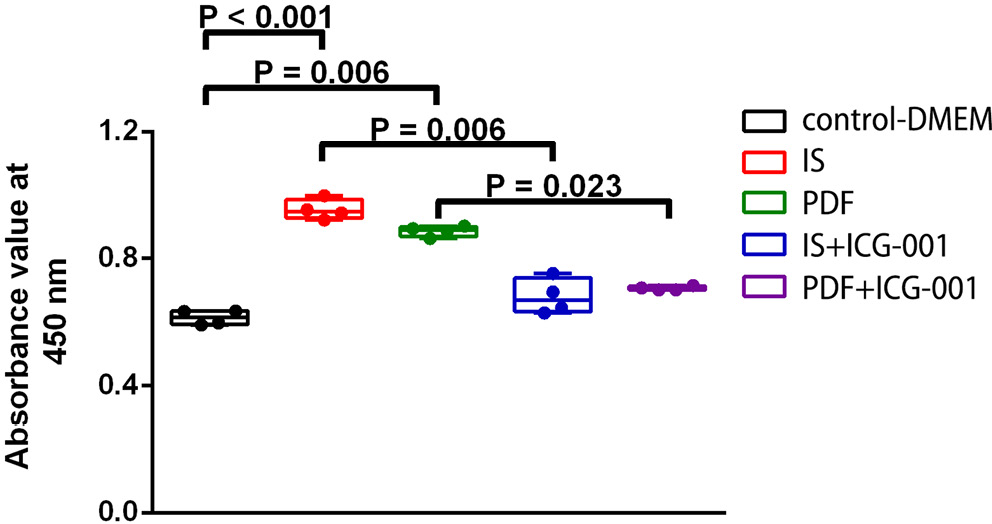
Cell Counting Kit-8 assay was used for evaluating proliferation status after IS treatment. Viable cell numbers showed significant differences between the control group and IS or PDF groups (p = 0.002 and p = 0.022, respectively), and between the IS and IS+ICG-001 and the PDF and PDF+ICG-001 groups (p = 0.008 and p = 0.041, respectively) at 72 h. No significant differences were found between the control group, IS+ICG-001 or PDF+ICG-001 groups at 72 h (Figure 2).
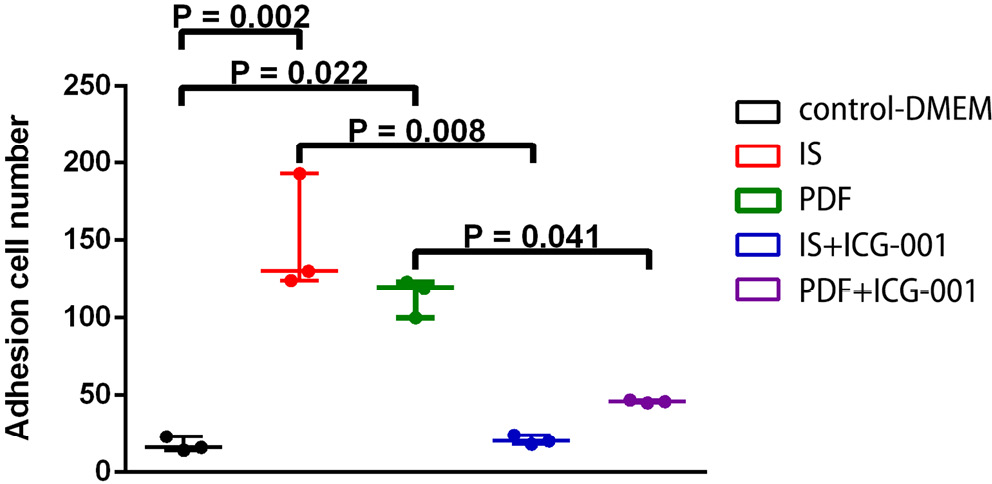
Matrigel was used for evaluating the adherence ability of cells after IS treatment. Adhesion cell numbers significantly increased after IS or PDF treatment compared with those of the control group (p < 0.001 and p = 0.006, respectively). ICG-001 significantly reduced the adhesion cell numbers compared with IS or PDF treatment only (p = 0.006 and p = 0.023, respectively) (Figure 3).
RNA expression of MMT markers
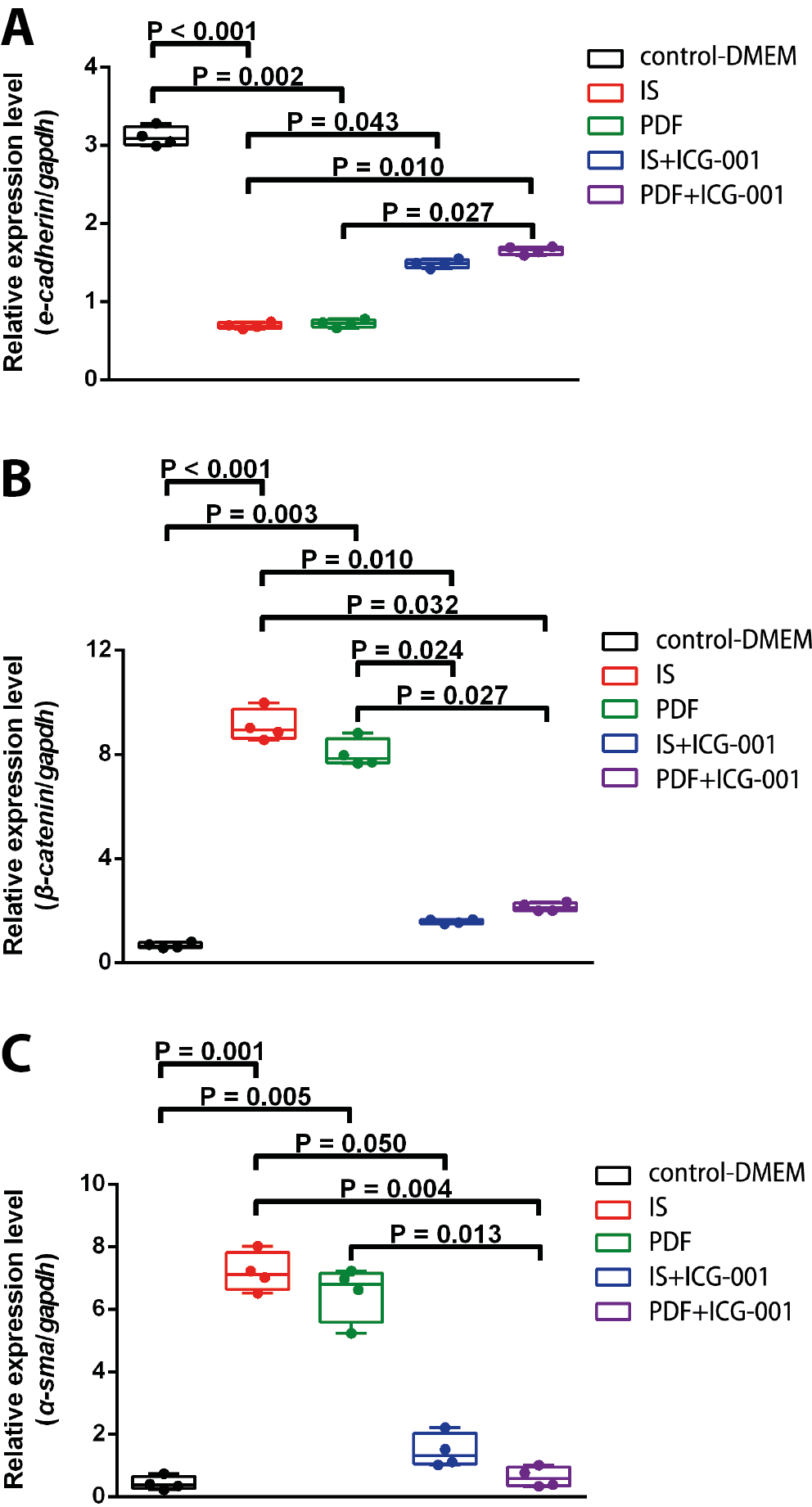
The present study sought to evaluate the RNA expression of E-cadherin, β-catenin and α-SMA in order to determine whether MMT was induced by IS and the related signaling pathway in HMrSV5 cells. After being treated by IS or PDF, E-cadherin RNA expression was significantly reduced compared with that of the control group from 12 to 72 h (data not shown). Adding ICG-001 reversed the change of E-cadherin RNA expression after IS or PDF treatment, shown by the significant differences in E-cadherin RNA expression between the IS and IS+ICG-001 groups at 48 h (Figure 4A, H = 17.5, p = 0.002). After being treated by IS or PDF, β-catenin RNA expression was significantly increased compared with that of the control group from 12 to 72 h (data not shown). Adding ICG-001 reversed the change of β-catenin RNA expression, as shown by the significant differences in β-catenin RNA expression between the IS and IS+ICG-001 groups, and PDF and PDF+ICG-001 groups at 72 h (Figure 4B, H = 18.0714, p = 0.001). After being treated by IS or PDF, α-SMA RNA expression was significantly increased compared with that of the control group from 24 to 72 h (data not shown). Adding ICG-001 reversed the change of α-SMA RNA expression, shown by the significant differences in α-SMA RNA expression between the IS and IS+ICG-001 groups and PDF and PDF+ICG-001 groups at 48 h (Figure 4C, H = 16.7733, p = 0.002).
Protein expression of MMT markers
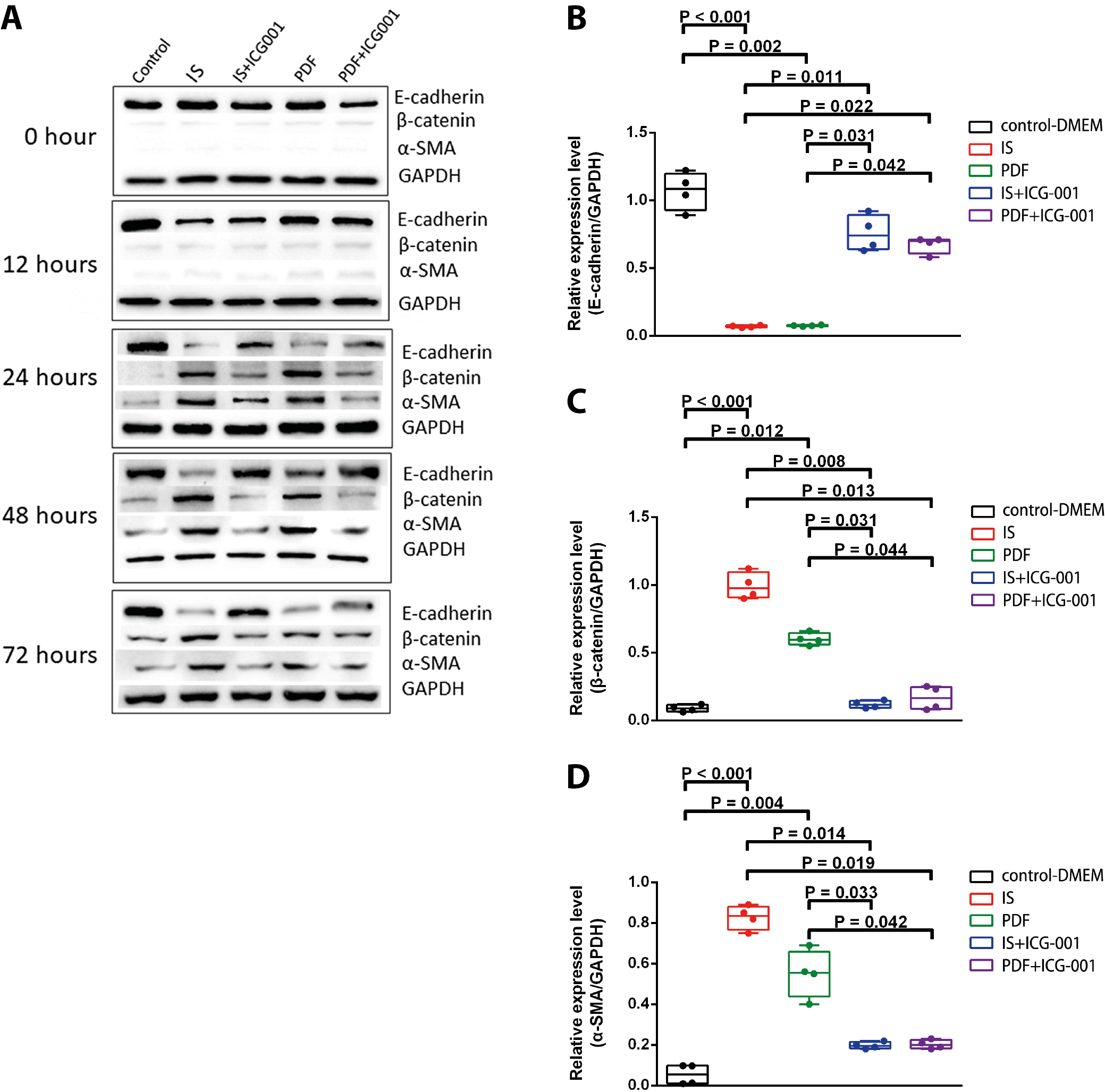
The protein expression of E-cadherin, β-catenin and α-SMA was evaluated. The representative images and quantitative data are shown in Figure 5A and 5B–5D, respectively. After being treated by IS or PDF, E-cadherin protein expression was significantly reduced compared with that of the control group from 24 to 72 h. Adding ICG-001 reversed the change of E-cadherin protein expression, shown by the significant differences in E-cadherin protein expression between the IS and IS+ICG-001 groups and PDF and PDF+ICG-001 groups at 48 h (Figure 5B, H = 16.4552, p = 0.002). After being treated by IS or PDF, β-catenin protein expression was significantly increased compared with that of the control group from 24 to 72 h. Adding ICG-001 reversed the change of β-catenin protein expression, shown by the significant differences in β-catenin protein expression between the IS and IS+ICG-001 groups, and PDF and PDF+ICG-001 groups at 48 h (Figure 5C, H = 15.744, p = 0.003). After being treated by IS or PDF, α-SMA protein expression was significantly increased compared with that of the control group from 24 to 72 h. Adding ICG-001 reversed the change of α-SMA protein expression, shown by the significant differences in α-SMA protein expression between the IS and IS+ICG-001 and PDF and PDF+ICG-001 groups at 24, 48 and 72 h (Figure 5D, H = 17.4119, p = 0.002).
Discussion
In the present study, IS was found to have the same capability as high-glucose PDF to induce MMT in human peritoneal mesothelial cells, which was presented by exhibiting a fibroblast-like appearance and functionally gaining the abilities of adhesion and proliferation. In addition, other MMT features including upregulated α-SMA and β-catenin, and downregulated E-cadherin in both protein and RNA levels were also found. These changes were reversed by the specific β-catenin inhibitor ICG-001, indicating that in peritoneal mesothelial cells, the β-catenin signaling pathway was not only involved in PDF-induced MMT, but IS-induced MMT was also activated through the β-catenin pathway.
Besides the convenience of implementation because it does not require frequent visits to hospital or medical facility, as well as assures greater patient mobility and higher quality of life for patients, PD is selected as the modality for RRT for better preservation of residual renal function, reduction of the risk of cardiovascular comorbidities or infection, and improvement of the prognosis of patients with ESRD rather than HD.16 The peritoneal cavity is covered by semipermeable peritoneum, which is composed of a sub-mesothelial zone, a basement membrane and a single layer of mesothelial cells as the first-line barrier. However, the integrity of the peritoneal mesothelial barrier can be disrupted after PD. The pathogenetic mechanisms are recognized including angiogenesis, hyalinizing vasculopathy and fibrosis.28, 29 Significant crosstalk and interplay between these changes and the associated signaling pathways have been demonstrated.30
The EMT plays a key role in embryogenesis and wound healing under normal physiological conditions and is typically activated following injury, where it is associated with organ fibrosis. Renal fibrosis-associated diseases include CKD, polycystic kidney disease and diabetic nephropathy. Peritoneal fibrosis due to PD can be considered as one of the consequences of renal fibrosis-associated diseases.25 Podocytes, endothelium and tubular epithelial cells in the kidney, and peritoneal mesothelial cells have been identified as sources of EMT/MMT during the renal fibrosis-associated disease progression, as described previously.25 The EMT can be triggered by various signaling pathways, many genes and protein may involve in this process, and these pathways crosstalk intensively and intricately. Transforming growth factor beta, Wnt and NECD are recognized as the main activated receptors of the injury stimuli, and they sequentially activate specific transcriptional molecules for the expression of mesenchymal and inhibitory function of endothelial proteins.25 Beta-catenin is the downstream molecule of both TGF-β and Wnt signaling pathways. As shown in a peritoneal fibrosis animal model, TGF-β treatment increased the expressions of WNT2 and WNT4, and also of β-catenin. Transforming growth factor beta treatment increased the expressions of WNT2 and WNT4, and also of β-catenin. When WNT antagonist DKK-1 or β-catenin inhibitor ICG-001 was treated, both resulted in attenuation of peritoneal angiogenesis; in addition, DKK-1 increased E-cadherin level and inhibited MMT, which all indicates the intensive crosstalk of the signaling pathways.31 Thus, β-catenin was chosen to be monitored in the present study. Transforming growth factor beta is a key contributor to peritoneal injury and is associated with the induction of collagen deposition, fibroblast activation and angiogenesis of the peritoneum.32 Signaling pathways induced by TGF-β divide into Smad-dependent and non-Smad pathways. In the Smad-dependent pathway, Smad2/3 is phosphorylated by PKC and activated by TGF-β receptor 1 and activin I-β; then, it is released from the receptor complex and binds to Smad4. This complex translocates to the nucleus to regulate the transcription of target genes involved in fibrosis. Via the non-Smad pathway, TGF-β activation leads to the inactivation of β-catenin inhibitor GSK-3β by phosphorylation. Elevated expressions of WNTs and β-catenin have been observed in isolated peritoneal mesothelial cells from PD patients, and are strongly associated with MMT.33 Through the same process of inactivation of GSK-3β by TGF-β or WNTs, released β-catenin translocates to the nucleus and activates the transcription of target genes including fibronectin, Twist, MMPs, Jagged1, LEF1, and Snail, which are the key factors during EMT/MMT development.25 For example, activated Snail directly inhibits E-cadherin expression, and matrix metalloproteinase-9 (MMP-9) cleaves E-cadherin.34, 35 Besides signaling transduction, β-catenin can bind with E-cadherin, and this complex is associated with actin filaments to form a dynamic link with the actin cytoskeleton, which is important for the maintenance of epithelial cell layers.33 Isolated peritoneal mesothelial cells from the effluent of patients underwent PD for more than 1 year had higher expression levels of β-catenin than that from patients who just started with PD for less than 1 month, and it was accompanied by expressional changes of E-cadherin and α-SMA, which are well-known markers of EMT/MMT, indicating the importance of β-catenin in MMT during PD.33 Thus, β-catenin was selected for the initial step to clarify the involved signaling pathway of IS-induced MMT in peritoneal mesothelial cells in the present study. In addition, in a peritoneal fibrosis mouse model, inhibition of β-catenin by ICG-001 showed reduced high-glucose induced angiogenesis severity and VEGF level of the peritoneum,31 so ICG-001 was used in the present study to confirm the involvement of β-catenin.
Indoxyl sulfate is an end product of dietary tryptophan, which is formed in liver and then enters systemic circulation.36 Indoxyl sulfate has been thought of as a consequent sign of declining renal function initially; however, emerging data indicate that IS has direct cytotoxicity on proximal tubular epithelial cells and negatively impacts the kidney, and IS exposure may be associated with CKD progress.22 Under healthy conditions, IS is trapped by organic anion transporters (OATs) at the basolateral membrane of proximal tubular epithelial cells and then excreted by active secretion, which may explain why previous studies have focused mainly on this type of renal cell. In the present study, the effects of IS on the peritoneum of patients with PD was evaluated since the peritoneum is used as dialysis membrane during PD, intensive contact of IS may be associated with MMT and fibrosis of the peritoneum. Despite this, IS has been found to be involved in fibrosis, a long-term process involving several cell types. For example, Nakano et al. demonstrated that IS induces EMT in the human tubular epithelial cell line HK-2, promotes the differentiation of the fibroblast cell line NRK-49F into myofibroblasts, and triggers an inflammatory response in the macrophage cell line THP-1, all through the mTORC1 signaling pathway.21 It remains unknown whether a specific cell type in the peritoneum selectively responds to IS. In this study, we provide evidence that exposure to IS triggers MMT in peritoneal mesothelial cells, potentially contributing to peritoneal injury during PD.
Based on preliminary test results, we selected a treatment concentration of 1 mmol/L for IS. Although this concentration was considerably lower than the serum levels observed in HD patients and even in healthy study participants, significant MMT features were still evident. Previous studies have indicated that serum IS levels in HD patients were more than 50 times higher than those in individuals with normal renal function (2.99 ±0.18 mg/dL or 140 mmol/L pre-HD vs 0.05 ±0.01 mg/dL or 2.34 mmol/L).37 As the primary contact layer, peritoneal mesothelial cells may activate signaling pathways in response to IS as an injury-induced stimulus. Consequently, the impact of IS exposure on these cells cannot be overlooked.
For effective treatment and prevention of peritoneal dysfunction in patients undergoing PD, it is essential to identify both the underlying mechanisms and the key inducers. Currently, high glucose exposure of peritoneal mesothelial cells during PD is the only known inducer, which has led to the development of glucose-sparing dialysis solutions to mitigate the risk of high glucose-induced MMT.38 Indoxyl sulfate, a uremic toxin, was identified as another potential inducer of MMT via the β-catenin signaling pathway during PD in the present study.
Therefore, routine monitoring of serum and peritoneal IS levels, along with the development of strategies to remove or reduce IS levels in the peritoneal cavity, could provide an alternative approach to prolonging PD use in patients with ESRD. Additionally, the use of specific inhibitors, such as ICG-001 (employed in this study to block the MMT signaling pathway), may offer potential benefits. The definition of abnormal serum and peritoneal IS level should be established by a larger survey including ESRD patients under HD or PD, and individuals with normal renal function. Additionally, effective and safe techniques for the repeated assessment of morphological and molecular changes in the peritoneum, such as endoscopic methods in both animal and clinical studies, should be developed to enable comprehensive and regular evaluations.
Limitations
The primary limitation of this study is that it was conducted exclusively in vitro. Additionally, to highlight the maximum pharmacological effects of the treatments investigated, results were presented at a single time point. More in vitro and in vivo studies should be done to overcome the limitations of the present study. For example, data of direct assessment of cell viability and vitality should be provided. Furthermore, to strengthen the association between IS and MMT, evidence from PD animal models and clinical patients are needed. Assessments of IS levels in the peritoneal cavity, morphological changes at the cellular and tissue levels, and the expression of molecules involved in β-catenin-associated signaling pathways should be performed in the future.
Conclusions
Indoxyl sulfate is known to be associated with EMT in proximal renal tubular cells. However, its impact on peritoneal mesothelial cells, which serve as the first-line barrier in PD, remains unexplored. Indoxyl sulfate induces MMT of human peritoneal mesothelial cells morphologically and functionally and these changes can be reversed by specific β-catenin inhibitor ICG-001, indicating β-catenin signaling pathway plays an important role in the process of MMT in peritoneal mesothelial cells. Routine monitoring of serum IS may be necessary to prolong PD survival and improve the outcome. Further investigation of the complete mechanism for developing strategy focused on IS-induced MMT might be worthy for patients who underwent PD.
Supplementary data
The supplementary materials are available at https://doi.org/10.5281/zenodo.14032147. The package includes the following files:
Supplementary Table 1. The results of post hoc Dunn’s test of the variables presented in Figure 2.
Supplementary Table 2. The results of post hoc Dunn’s test of the variables presented in Figure 3.
Supplementary Table 3. The results of post hoc Dunn’s test of the variables presented in Figure 4A.
Supplementary Table 4. The results of post hoc Dunn’s test of the variables presented in Figure 4B.
Supplementary Table 5. The results of post hoc Dunn’s test of the variables presented in Figure 4C.
Supplementary Table 6. The results of post hoc Dunn’s test of the variables presented in Figure 5B.
Supplementary Table 7. The results of post hoc Dunn’s test of the variables presented in Figure 5C.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.