Abstract
Background. The impact of different systemic treatments on the health-related quality of life (HRQoL) in patients with metastatic colorectal cancer (mCRC) is still unclear.
Objectives. To compare and evaluate the effects of various systemic interventions on the HRQoL in patients with mCRC.
Material and methods. A thorough search was conducted using four electronic databases (PubMed, Embase, Scopus, and Cochrane Library) to locate relevant literature published in peer-reviewed journals. The risk ratio (RR) and 95% confidence intervals (95% CIs) were calculated. The heterogeneity was examined using p-value, Cochrane Q and I² statistics. The analysis was performed with RevMan 5.4. At least 2 treatment regimens were tested in phase II or III trials. The primary objectives were short- and long-term mean changes in EORTC QLQ-C30 GHS/QoL (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30, Global Health Status/Quality of Life) and EQ-5D health utility scores (EuroQol 5 Dimension). Multivariate meta-regression was used to combine direct and indirect comparison data into a network meta-analysis with a random-effects consistency model. The surface under the cumulative ranking (SUCRA) probability curve was used to compare different systemic therapy combinations.
Results. This meta-analysis involved 15 relevant randomized clinical trials (RCTs) with 7,699 patients with mCRC. The study had a low risk of bias (RoB) (p > 0.05 for Egger’s regression test) and moderate heterogeneity (I2 < 60%). Results indicated that systemic therapies were substantially more effective than other agents in improving the overall survival (OS) of patients (RR: 0.85 (95% CI: 0.79–0.90); p < 0.001, I2 < 60%], ensuring progression-free survival (PFS) (RR 0.80 (95% CI: 0.75–0.85); p < 0.001; I2 < 60%), suggesting that there was moderate heterogeneity. Long-term findings demonstrated that cetuximab was the most effective treatment and was linked to a significant improvement in GHS/QoL.(coefficient [95% CI] = 0.23 [−0.68 to 0.96], p = 0.747). In terms of the long- and short-term results of change in QLQ-C30 GHS/HUS QoL score, cetuximab performed the best (SUCRA 95.12%) among all therapies. It also showed a substantial advantage in comparison to chemotherapy (mean deviation (MD) 0.06, 95% CI: 0.01 to 0.09).
Conclusions. This network meta-analysis found that cetuximab monotherapy improves HRQoL and prolongs OS and PFS in patients with mCRC.
Key words: colorectal cancer, metastasis, health-related quality of life, immunotherapies, patient-reported outcome
Introduction
Colorectal cancer (CRC) is a malignancy that specifically targets the colon, the large intestine or the rectum. It has the potential to inflict significant damage and result in fatalities. The risk of CRC increases with age.1 Colorectal cancer is the 3rd most prevalent cancer worldwide and is responsible for the 3rd greatest number of cancer-related deaths.2 Screening can identify initial instances of CRC, which manifest as benign polyps without any symptoms. Colorectal cancer symptoms may vary depending on the size and location of the tumor. However, most people notice changes in their bowel habits, changes in their stool consistency, the presence of blood in their stool, and abdominal discomfort.3, 4 Metastatic colorectal cancer (mCRC) occurs in around 25% of all patients, and around 50% of individuals without metastases will eventually develop metastasis.5
Factors such as the tumor’s size and location and extent of metastasis determine the CRC treatment. Typical treatment methods consist of surgical removal of the malignancy, systemic therapy (such as chemotherapy, immunotherapy, hormone therapy, or targeted therapy) and radiation therapy.6, 7, 8 Systemic therapies are medications that target and eradicate cancer cells across the entire body, regardless of their location. The most commonly used systemic treatments for CRC are irinotecan, cetuximab, bevacizumab, and panitumumab.9, 10, 11 The longevity of patients with mCRC has recently increased as a result of breakthroughs in targeted therapy. The 3-year survival rate for patients with mCRC has surged from 25% to 30% over the course of a decade.12 Several randomized clinical trials (RCTs) have investigated different systemic interventions for the mCRC management.13, 14, 15 Nevertheless, the efficacy results of those RCTs have been inconsistent, which makes it difficult to draw a definitive conclusion regarding the preferred strategy.
With the improvement in survivability rates and treatment choices, it has become critical to prioritize a satisfactory HRQoL alongside extending life expectancy. Therefore, researchers evaluated HRQoL as a measure of patients’ self-reported outcomes to assess the impact of different systemic interventions on patients’ health. There are 5 areas of quality of life (QoL) that HRQoL looks at: physical, role, cognitive, emotional, and social functioning. Health-related quality of life uses 2 different scales: the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Core 30, Global Health Status/Quality of Life (EORTC QLQ-C30 GHS/QoL) general health status and QoL questionnaire and the EuroQoL-five-dimension index questionnaire (EQ-5D).16
The EORTC QLQ-C30 questionnaire includes a global health status and quality of life (GHS/QoL) scale based on a 7-point Likert scale from “very poor” to “excellent,” and the EQ-5D questionnaire, which includes the visual analogue scale (VAS) and health utility scores (HUS).17 Nevertheless, there is a lack of research comparing the HRQoL of patients with mCRC across various systemic interventions. Therefore, we conducted a network meta-analysis (NMA) of 15 RCTs,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 which were selected in accordance with pre-specified inclusion and exclusion criteria, to comprehensively compare the impacts of various systemic interventions on HRQoL in patients with mCRC and to compile relevant references for patients, clinicians and cancer management guidelines.
Objectives
The rationale of this systematic review and network meta-analysis was to compare and evaluate the effects of various systemic interventions on the HRQoL in patients with mCRC.
Materials and methods
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extensions for NMA.33
Eligibility criteria
The following eligibility criteria were implemented using the PICOS (Population, Intervention, Comparison, Outcomes, and Study) framework: The Population of this study included adult patients who diagnosed with advanced or metastatic unresectable CRC, based on either histological or cytological confirmation. There were no restrictions placed on individual-level characteristics. All systemic approaches, such as pharmacological, surgical, radiological, and combination therapies, were included in the analyzed interventions and comparisons. Included RCTs should have reported at least 1 of the following outcomes as the primary clinical outcome: EORTC QLQ-C30 GHS/QoL score, EQ-5D VAS and HUS. We selected the EORTC QLQ-C30 and EQ-5D as assessments of HRQoL frequently included in RCTs encompassing mCRC. The GHS/QoL subscale from the EORTC QLQ-C30 questionnaire has a standard range of values from 0 to 100. A high score on the GHS/QoL scale indicates excellent global health status and a higher QoL, and vice versa. A change of 5 to 10 points on the GHS/QoL scale is accepted as the minimum clinically important difference (MCID) for GHS/QoL.
Information sources
To identify pertinent RCTs and published studies, we implemented an exhaustive search of PubMed, Scopus, Embase, and Cochrane Library by May 31, 2024. The key words that we used were “colorectal cancer,” “systemic treatment,” “metastatic colorectal cancer,” “advanced colorectal cancer,” “health-related quality of life,” “quality of life,” “QoL,” “patient reported outcome,” “colorectal carcinoma,” “treatment section,” “network meta-analysis,” “systematic review,” “systematic review and meta-analysis,” “meta-analysis,” “RCT,” and “randomized controlled trial.” Table 1 outlines the search strategy.
Search strategy
During the Scopus search, the specified key words were entered into the Title (ti)-Abstract (abs)-Keyword (key) field. The Cochrane search terms encompassed “metastatic colorectal cancer” and “systemic interventions.” We employed the PICO framework34 to establish precise selection criteria. In this context, “P” refers to patients diagnosed with colorectal cancer, “I” represents the inclusion of multiple systemic interventions, “C” represents a control group, and “O” encompasses the enhancement of clinical outcomes, specifically the improvement in HRQoL. During the course of this investigation, the research strategy was restricted to the utilization of RCTs. The inclusion criteria required that only papers written in English were considered. This was done to guarantee the accuracy of the data interpretation and analysis, taking into account the expertise of the research team. Furthermore, there were no publication date restrictions.
Selection process
The titles and abstracts of the included articles were initially reviewed by 2 researchers. This study was primarily designed using the phase II or III clinical trials, which examined multiple distinct treatments. For the purpose of eliminating redundancy, we exclusively included studies that yielded the most up-to-date and enlightening results and excluded studies that were not relevant to any comparisons or investigated different doses with the same combinations.
Data extraction
Two researchers independently extracted the pertinent data. Discrepancies were resolved in collaboration with other investigators. The retrieved information included following specific characteristics of trials that met the eligibility criteria: study ID and year, journal of publication, country of the study, total number of participants, number of participants in the intervention arm, number of participants in the control arm, intervention medications, control medications, age of participants, sex (M/F), line of treatment, and primary outcomes. Clinical outcomes analyzed were mean change from baseline in EORTC QLQ-C30 GHS/QoL and EQ-5D HUS scores.
Study risk of bias assessment
The Cochrane Collaboration’s risk of bias (RoB) tool35 was used to evaluate the quality of the included studies. The qualifying studies were classified into 3 categories: high, low or uncertain risk. The existence of publication bias was evaluated with a funnel plot36 and the application of the Egger’s regression test.37 Any result with a p-value of less than 0.05 was considered evidence of bias.38
Synthesis methods
The impact of the intervention on EORTC QLQ-C30 GHS/QoL scores in the short and long term and the mean change in EQ-5D HUS from baseline to endpoint were the primary outcomes. The long-term period was defined as the time from baseline to the endpoint, while the short-term period was defined as a period of 8–12 weeks from baseline. The data from the included trials were used to calculate the risk ratio (RR) and 95% confidence interval (95% CI)39 for overall survival (OS) and progression-free survival (PFS) of patients receiving the intervention treatment compared to the control group using the DerSimonian and Laird random-effects model.40 A random-effects consistency model41 was implemented to conduct a network meta-analysis. This involved employing a multivariate meta-regression technique42 to combine data from both direct and indirect comparisons. The network meta-analysis maintained the transitivity assumption, which ensured that interventions were comparable. The coherence between direct and indirect evidence was evaluated using node-splitting methods, and any inconsistencies were carefully examined for methodological or clinical reasons. The I2 statistic was used to assess the heterogeneity of the included studies. A value greater than 50% indicated a moderate degree of heterogeneity.43 A two-tailed test with a p-value less than 0.05 was considered statistically significant.44 In addition, treatment solutions were evaluated and graded based on the surface under the cumulative ranking (SUCRA) probability.45 A greater efficacy was associated with higher SUCRA scores. The SUCRA values were used to determine the relative ranking of treatments. The scores, which ranged from 0 to 100%, represented the likelihood that each treatment was the most effective option within the network. Higher values indicated superior relative performance.
Results
Literature search results
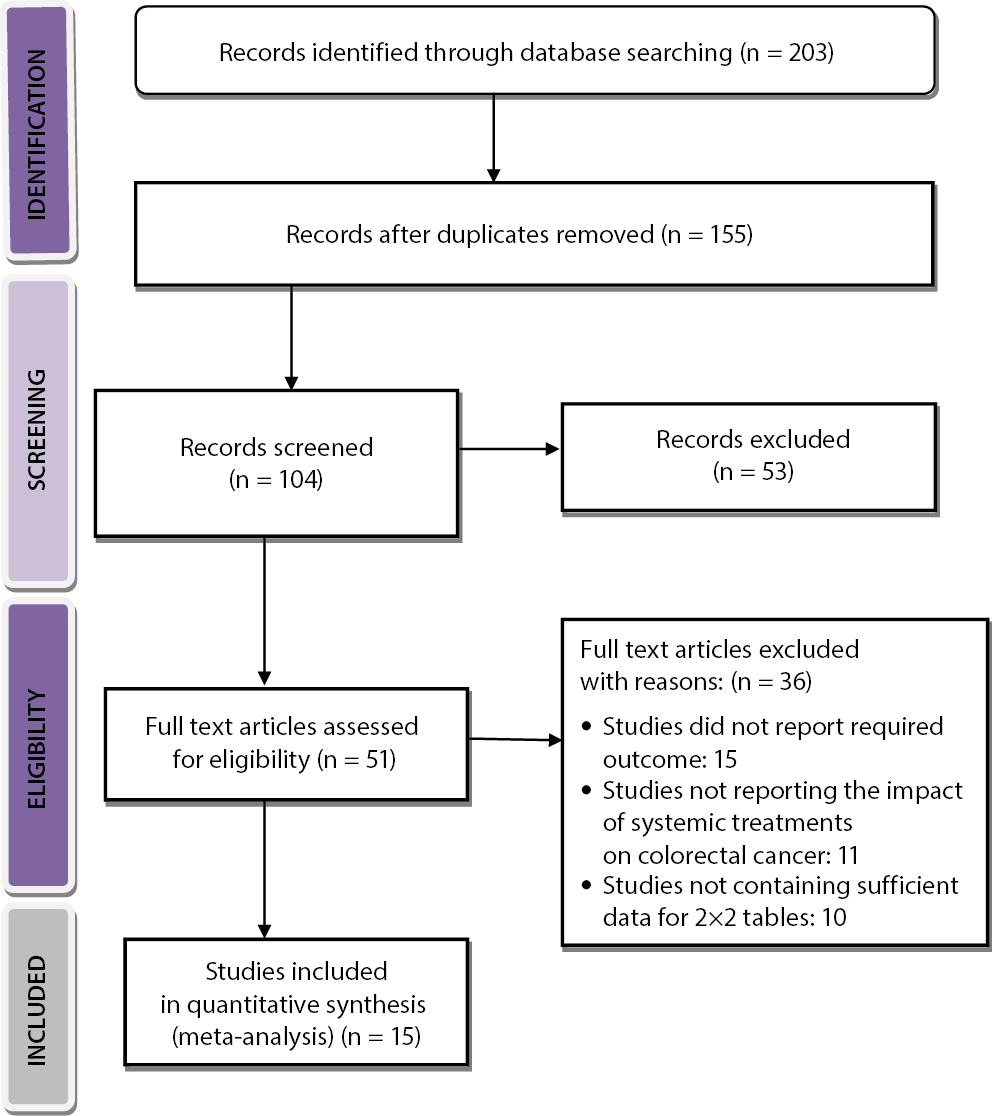
A comprehensive electronic search of several databases was carried out and a total of 203 articles were identified that met the inclusion and exclusion criteria as indicated in the PICOS. A total of 48 entries were excluded due to their duplication, and 155 records were identified. Subsequently, 104 records underwent review, and 51 were excluded due to the presence of invalid titles and abstracts. Following the completion of complementary screening, a total of 51 records were assessed to determine their eligibility. However, after applying the established criteria for including and excluding studies, 36 studies were found to be ineligible and were therefore excluded. The studies were excluded mainly because they did not meet the criteria for inclusion, did not provide sufficient data to create 2×2 tables and did not have relevant outcome measures. Finally, 15 RCT studies that met the specified inclusion criteria and were published between 2000 and 2024 were included in this review and meta-analysis, as illustrated in Figure 1.
This meta-analysis is a comprehensive examination of 7,699 individuals diagnosed with CRC, spanning a variety of age groups. The efficacy of various therapeutic agents, including bevacizumab, panitumumab, cetuximab, FOLFOX, CAPOX, irinotecan, fluorouracil, leucovorin, and folinic acid, was evaluated in the included RCTs. The main characteristics of the studies included in this meta-analysis are shown in Table 2. The meta-analysis was subsequently conducted using the aforementioned event data.
Risk of bias assessment
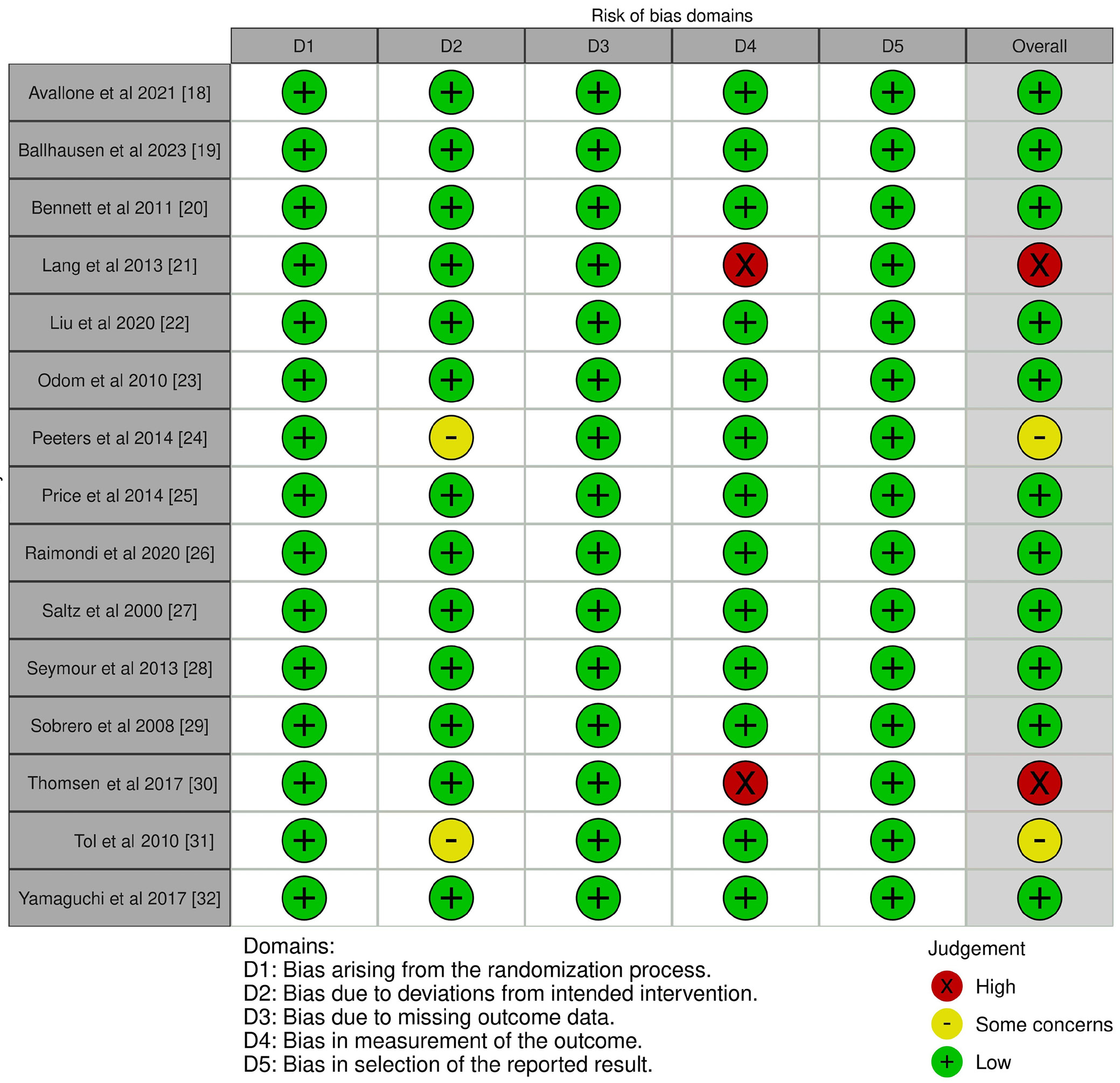
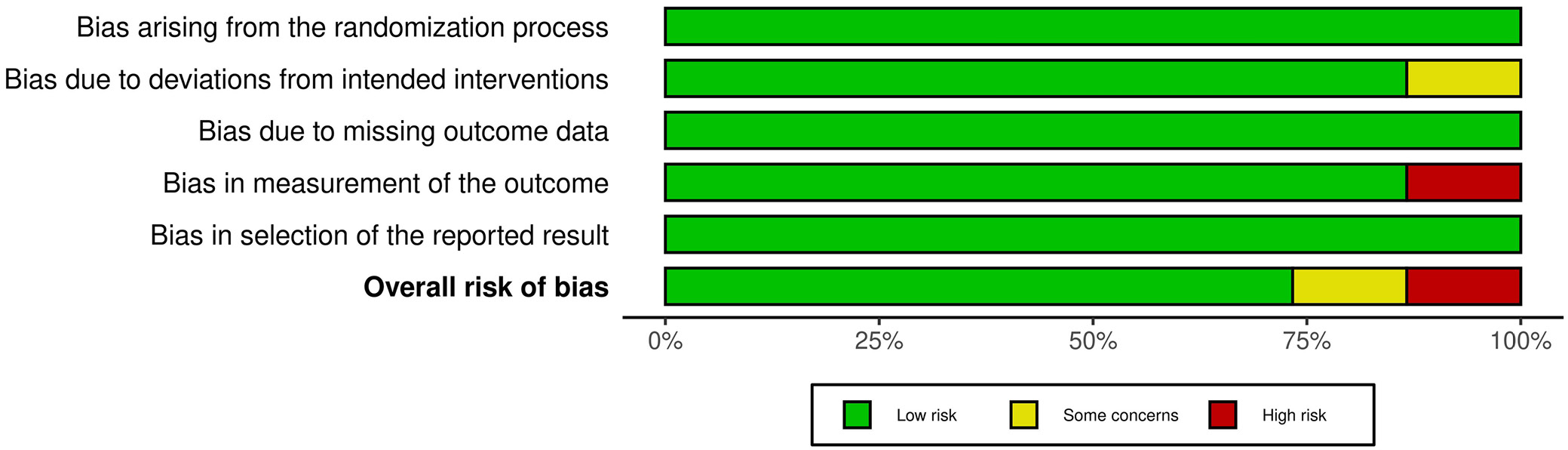
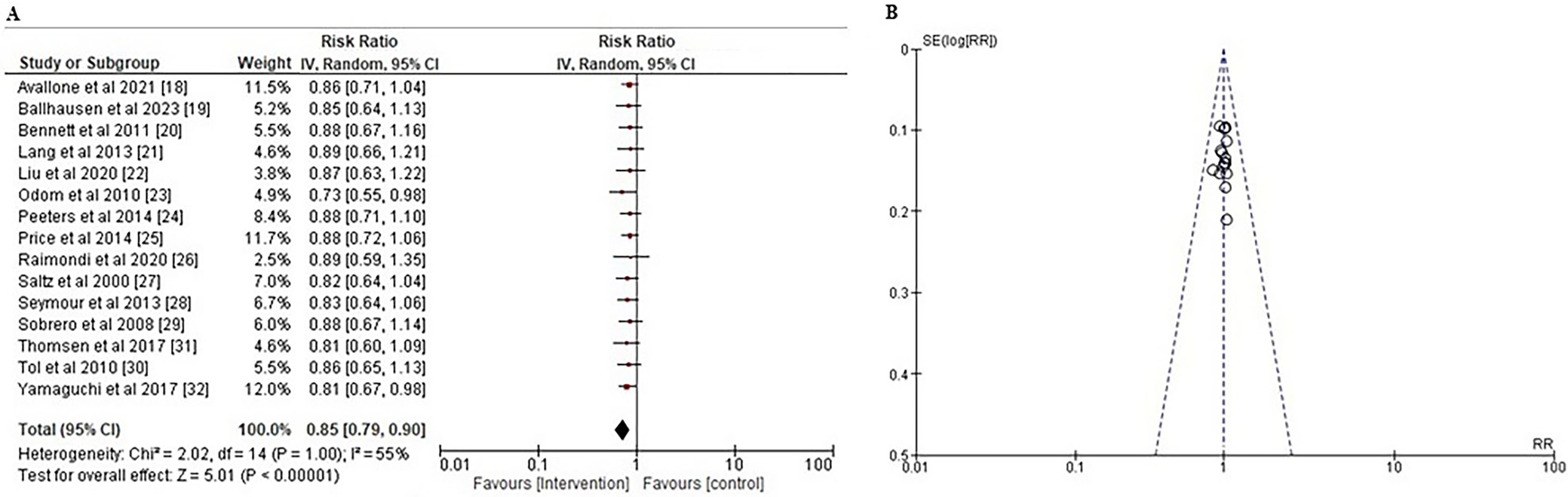
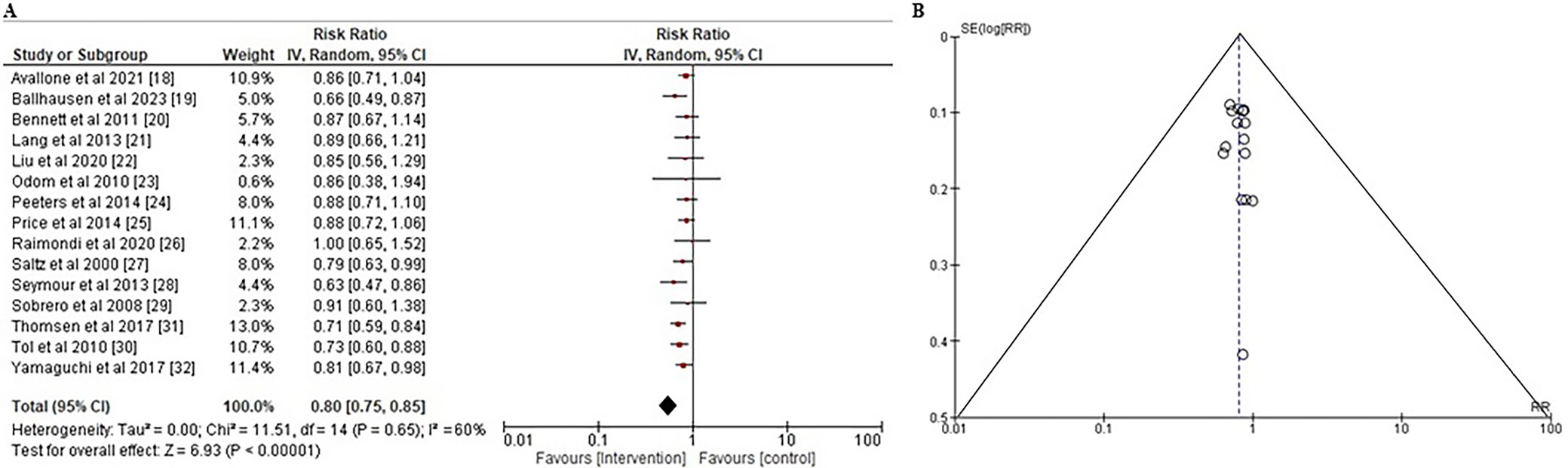
To ascertain the study’s overall quality rating, we implemented an evaluation of the potential RoB. The results of the RoB assessment for each of the 15 included RCTs are presented in Table 3, which was conducted using the pre-established questionnaire. According to the traffic light plot in Figure 2 and the summary plot for bias assessment in Figure 3, the current meta-analysis presents a low RoB. We discovered that 11 of the 15 RCTs had a low RoB. However, 2 RCTs conducted by Peeters et al.24 and Tol et al.31 demonstrated a moderate degree of bias resulting from deviations from the intended intervention. Lang et al.21 and Thomsen et al.30 conducted 2 additional RCTs that had a substantial RoB in outcome measurement.
Findings of the statistical analysis
The current meta-analysis included 7,699 patients with CRC from 15 selected RCTs to evaluate the impact of different systemic interventions on HRQoL in patients with mCRC. The following conclusions were obtained from the statistical analysis of the primary study outcome:
The impact of various systemic interventions on patients’ overall survival rate
Measuring the OS of patients in a clinical trial is a reliable method to assess the effectiveness of a newly developed medicine. Overall survival in the context of cancer treatments refers to the duration of time from diagnosis or the initiation of treatment for cancer until death.46, 47 To assess the effect of various systemic interventions on the OS of patients with CRC, the RR, along with the 95% CI based on the event data extracted from the included studies, was calculated as shown in Figure 4A. Results indicated that systemic therapies were substantially more effective than other agents in improving the OS of patients with a risk ratio less than 1 (RR: 0.85 (95% CI: 0.79–0.90); χ2 = 2.02, degrees of freedom (df) = 14, Z = 5.01, and p < 0.001). The I2 value of 55% indicated moderate heterogeneity. In addition, the symmetrical funnel diagram shown in Figure 4B and the statistically insignificant p statistic of Egger’s test (p = 0.262), which is higher than the predetermined significance threshold of 0.05, indicate a low probability of publication bias.
The impact of various systemic interventions on progression-free survival of patients
Progression-free survival refers to the time after surgical removal of the tumor and the first signs of tumor recurrence or disease progression.48 To evaluate the impact of a variety of systemic interventions on the PFS of CRC patients, the RR and the 95% CI were calculated using the event data extracted from the included studies, as illustrated in Figure 5A. The findings showed that systemic interventions were significantly more successful than other treatments in ensuring PFS with a RR of less than 1 (RR: 0.80 (95% CI: 0.75–0.85); χ2 = 11.51, df = 14, Z = 6.93, and p < 0.001). The I² value of 60% indicated of moderate heterogeneity. Moreover, the symmetrical funnel plot illustrated in Figure 5B, in conjunction with the statistically insignificant p-value of Egger’s test (p = 0.327), above the preset significance threshold of 0.05, suggested a minimal likelihood of publication bias.
Results of network meta-analysis
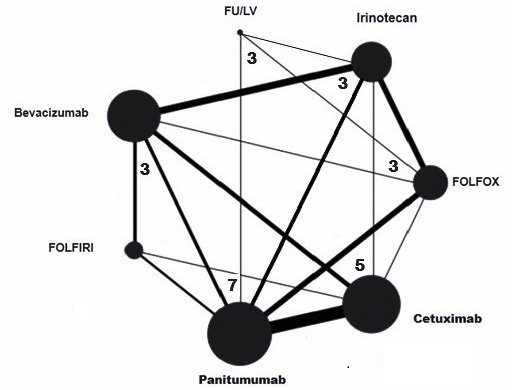
A NMA was conducted to evaluate the efficacy of various systemic interventions for CRC. This analysis entailed the synthesis of data from the included RCTs, which included both direct and indirect evidence. Figure 6 illustrates a network depiction that illustrates the relationships and interactions between various medications, as well as a collection of network estimates that illustrate the effects of various systemic interventions for all fundamental comparisons. The network graph is represented by nodes or vertices, which represent the total number of interventions. Edges or lines are used to represent the total number of direct comparisons between the nodes of the network. The number of studies represents the total number of studies included in the network. The degree of a node is the number of edges incident to the node, with loops counted twice.49
We observed a consistent correlation between the direct and indirect evidence while conducting the pairwise meta-analyses and analysing the data. No significant divergences between direct and indirect estimates were observed during the node splitting analysis, as all p-values in the inconsistency test were greater than 0.05. The consistency and inconsistency models did not exhibit any discernible distinctions. There was no significant difference in the consistency and inconsistency models for the improvement in OS and PFS using systemic interventions such as FOLFOX, bevacizumab, panitumumab, cetuximab, FOLFIRI, irinotecan, fluorouracil, leucovorin, and folinic acid (χ2 = 0.53, p = 0.854).
Furthermore, our results did not differ significantly between direct and indirect evidence: 1) for panitumumab vs control (coefficient [95% CI] = −0.06 [−0.47 to 0.72], p = 0.631), 2) for cetuximab vs control (coefficient [95% CI] = 0.23 [−0.68 to 0.96], p = 0.747), 3) bevacizumab vs control (coefficient [95% CI] = −0.42 [−1.87 to 1.46], p = 0.681), or 4) irinotecan vs control (coefficient [95% CI] = −0.34 [−1.54 to 1.31], p = 0.549). Long-term results showed that cetuximab performed best and was associated with significantly improved GHS/QoL.
Cumulative ranking of different systemic treatments for colorectal cancer
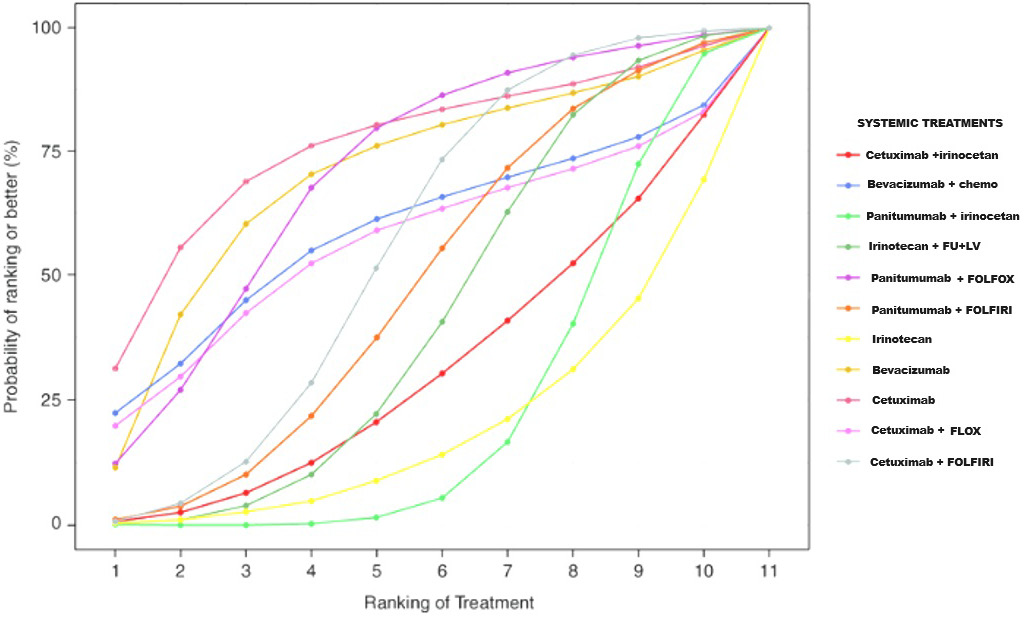
The SUCRA curve was developed to create a hierarchy of systemic therapies that considers the variability of their respective treatment effects. The SUCRA value quantifies the average rank and posterior probability of each medicine being among the top n alternatives. A higher SUCRA rating indicates a superior ranking for the therapy. The SUCRA curve in Figure 7 compares the effectiveness of various systemic interventions, including FOLFOX, bevacizumab, panitumumab, cetuximab, FOLFIRI, irinotecan, fluorouracil, leucovorin, folinic acid, and their combinations. The results indicate that cetuximab had the highest performance (SUCRA 95.12%) in improving both short-term and long-term QLQ-C30 GHS/HUS QoL scores. Furthermore, cetuximab showed a significant advantage over chemotherapy (MD 0.06, 95% CI: 0.01–0.09).
Discussion
Our findings indicate that there is a significant advantage in continuing the full induction regimen until disease progression, as opposed to periods of observation or maintenance therapy, in terms of both PFS and OS. Furthermore, our findings indicate that cetuximab offers a substantial benefit compared to alternative control treatments in terms of prolonging the time before disease progression and improving OS rates. An epidermal growth factor receptor inhibitor medication, cetuximab, is used to treat mCRC. It is marketed under the brand name Erbitux. Cetuximab is administered intravenously as a chimeric monoclonal antibody.50 In general, the preferred maintenance treatment appears to be cetuximab monotherapy or a combination of cetuximab and bevacizumab. Both single-agent panitumumab and bevacizumab seem to be viable choices. Monotherapy has demonstrated significant improvements in patient-reported HRQoL compared to combination therapy and immunotherapy and targeted therapy. Immunotherapy and targeted therapy showed the best results in terms of HRQoL.51, 52, 53 The addition of targeted therapy to chemotherapy did not improve patient performance in terms of HRQoL. The results demonstrated that cetuximab was highly effective in improving HRQoL in the management of mCRC, particularly when the baseline gene expression was not specified. This was particularly the case in patients who had previously undergone systematic treatment. A review of the overall impact on patients’ QoL, effectiveness and safety indicated that cetuximab is a beneficial treatment for patients who have wild-type Kirsten rat sarcoma virus (KRAS) mutations. The sensitivity analysis confirmed the robustness of the main results. After differentiating between chemotherapy medicines and removing RCTs with inconsistent baseline characteristics, the overall conclusions remained unaltered. Studies have been conducted to determine the most effective length of first-line induction chemotherapy in mCRC following an initial response or stable illness. These studies showed that it is possible to take a “management break” and then resume the same treatment when the disease progresses. Our study confirms that maintenance therapy with systemic treatment is a viable alternative to continuing the full course of treatment.
One significant benefit of reducing treatment intensity after attaining the highest level of response (usually within 3–4 months) with initial therapy is the reduction of harmful side effects, enhancement of overall wellbeing and potential cost improvement. The included RCTs clearly showed that patients who received maintenance treatment had lower rates of adverse effects than those who received the continuous induction regimen. These non-cytotoxic biologic medicines enable the preservation of benefits while further minimizing toxic effects. The use of cetuximab, panitumumab and bevacizumab has led to improved outcomes when incorporated into initial cytotoxic induction regimens for patients with mCRC. Although cost-effectiveness analysis has its limitations, it can provide valuable information to support shared decision-making with the patient in choosing the most effective maintenance approach. However, the pairwise comparison in the meta-analysis showed that this improvement was statistically significant for each of them compared with observation.
Earlier meta-analyses investigated the effectiveness of continuous vs intermittent chemotherapy approaches in patients with mCRC by analyzing data from RCTs.54, 55, 56 The results of this study suggest that intermittent methods do not lead to a clinically meaningful reduction in OS. Conversely, our study employed a network meta-analysis approach, exclusively encompassing RCTs that employed combination induction chemotherapy. The findings suggest that systemic interventions represent an established treatment protocol for patients with mCRC. Nevertheless, biomarkers are an indispensable tool in future research, facilitating the implementation of precision medicine, identification of distinct patient subgroups, optimization of treatment strategies, prediction of disease progression, and early detection of responses to therapy. Biomarkers play an essential role in risk assessment, enabling clinicians to personalize interventions and enhance patient outcomes. Therefore, integration of biomarkers in research paradigms will revolutionize patient care, yielding personalized and targeted treatments that maximize efficacy while minimizing adverse effects.57 Likewise, to move personalized medicine forward, make early intervention easier and improve therapeutic efficacy, more research needs to be done on figuring out predictive biomarkers and reducing side effects. By identifying and characterizing biomarkers, medical practitioners can implement precision-based treatment plans that minimize the risk of harm to patients and enhance patient safety and outcomes, thereby advancing the frontiers of precision medicine.58 The integration of biomarkers in clinical practice for systemic immunotherapies in CRC will enable personalized treatment planning, enhanced patient selection and real-time monitoring. This will optimize treatment outcomes, reduce toxicity and streamline clinical trials, ultimately revolutionizing CRC management with more effective and adaptive treatment strategies.59, 60
Limitations
Our study has limitations when it comes to network analysis and individual trials. Our initial analysis was conducted using data at the study level, rather than individual patient data, which restricted the effectiveness of our research. In addition, the lack of blinding of outcome assessors and the need for cautious interpretation of PFS in clinical trials pose a potential risk of detection bias. Furthermore, the majority of included RCTs did not differentiate between mutation types and gene expression levels when reporting HRQoL, so it was not possible to make indirect comparisons based on mutation target or patient gene expression levels. Moreover, the HRQoL-related data in this investigation primarily came from populations where the baseline expression levels of patients were not specified. Hence, it is necessary to get further clinical evidence to confirm the efficacy of various therapy methods in populations with precise levels of target expression. The lack of data on physical functioning, social functioning and tiredness ratings limited the ability to examine more complex components of HRQoL.
Conclusions
Immunotherapies and targeted medicines provide the best results in terms of improving patient HRQoL, but complicated therapy regimens are not as beneficial. Monotherapy, particularly with cetuximab, substantially improves HRQoL and provides a longer duration of OS and PFS compared to other control treatments. Patients with mCRC who have achieved stable disease after a certain length of induction treatment (3–4 months) are advised to undergo a maintenance schedule using cetuximab. However, the selection of the most efficient maintenance solutions should be based on considerations such as patient preference, the cost of treatment and the possibility for harmful consequences.