Abstract
Background. The motor symptoms in patients with Parkinson’s disease (PD) are commonly preceded by gastrointestinal (GI) symptoms. The enteric nervous system (ENS) has also been reported to exhibit neuropathological characteristics of PD.
Objectives. To evaluate the relationship between the incidence of parkinsonism and alteration in gut microbiota and pathogens.
Materials and methods. Studies in different languages that evaluate the relationship between gut microorganisms and PD were included into this meta-analysis. The outcomes of these studies were analyzed using a random effects model; it was also used to calculate the mean difference (MD) with 95% confidence interval (95% CI) in order to quantify the impact of different rehabilitation techniques on clinical parameters. Dichotomous and continuous models were used for the analysis of extracted data.
Results. A total of 28 studies were included in our analysis. The analysis of small intestinal bacterial overgrowth showed a significant correlation with Parkinson’s subjects compared with controls (p < 0.001). In addition, the presence of Helicobacter pylori (HP) infection was significantly related to the Parkinson’s group (p < 0.001). On the other hand, there was a significantly higher abundance level of Bifidobacteriaceae (p = 0.008), Verrucomicrobiaceae (p < 0.001) and Christensenellaceae (p = 0.003) in Parkinson’s subjects. In contrast, a significantly lower abundance levels in Parkinson’s subjects were found in Faecalibacterium (p = 0.03), Lachnospiraceae (p = 0.005) and Prevotellaceae (p = 0.005). No significant difference was related to Ruminococcaceae.
Conclusion. Parkinson’s subjects showed a higher degree of alteration of gut microbiota and pathogens compared with normal human subjects. Future multicenter randomized trials are needed.
Key words: intestinal bacteria, Helicobacter pylori, microbiota, Parkinson’s, gut
Introduction
Parkinson’s disease (PD) is a neurological movement illness that affects multiple systems and worsens over time.1 Patient with PD has both motor and nonmotor symptoms, such as hyposmia, sleep difficulties, depression, and gastrointestinal (GI) symptoms. Motor symptoms include resting tremors, bradykinesia, rigidity, and gait abnormalities.2 The most prevalent GI symptom in PD is constipation, which may be present in up to 80% of patients before the appearance of motor symptoms.3 Therefore, constipation is considered a clinical indicator for the diagnosis of prodromal PD.4 The loss of dopaminergic neurons in the substantia nigra pars compacta and the appearance of Lewy bodies (LBs) or Lewy neurites, which consist of aberrant α-synuclein aggregates, are the major neuropathological hallmarks of PD.5 According to Braak’s pathological staging, the development of PD begins with the ingestion or inhalation of a pathogen, which then causes the creation of LBs and their subsequent migration from the GI tract to the central nervous system via the vagus nerve.6 As a result, researchers began to pay more attention to the “gut–brain axis” as a potential player in the pathogenic mechanism of PD.
The presence of an intestinal infection has been associated with a higher risk of developing PD,7, 8 which may then lead to PD-like symptoms.9 The gut microbiota of PD patients has been shown to exacerbate α-synuclein-mediated motor impairments and brain disease in a PD mouse model, whereas a germ-free PD mouse model had less severe α-synuclein pathology.10 Accordingly, a disruption in the gut flora may be a risk factor for PD. The gut microbiota is an intricate system that produces several antimicrobial chemicals and serves as a physical barrier against invading infections.11 There is mounting evidence that LB development in the enteric nervous system (ENS) may be triggered by abnormalities in the gut microbiota and their metabolic products.
Intestinal epithelial Helicobacter pylori (HP) is a Gram-negative bacteria that, in the absence of eradication drug therapy, causes chronic mucosal inflammation and persistent infection.12 Studies conducted in the 1980s proved the causative association of this bacterium with peptic ulcers and stomach cancer.12 Subsequent research with varying levels of evidence confirmed the association between HP infection and several extra GI diseases, such as idiopathic thrombocytopenic purpura, unexplained iron deficiency anemia, ischemic heart diseases, and neurological disorders (such as stroke, Alzheimer’s disease and PD).13 Moreover, it has been investigated whether or not HP infection is linked to PD. Patients with PD, for instance, have been found to have a greater frequency of HP infections than the general population.8 In several case-control studies, patients with PD have been shown to have greater titers of antibodies to HP than controls.14 In addition, motor performance is worse in PD patients with HP infection compared to PD patients without HP infection,15 but motor function can be improved by changing levodopa absorption in PD patients who undergo HP eradication therapy.16 However, there has been a dearth of research on whether HP infection is linked to PD in the general population.
Objectives
The current meta-analysis aims to evaluate the relationship between the incidence of parkinsonism and alteration in gut microbiota and pathogens.
Materials and methods
Study design
Meta-analyses of clinical studies were included in the epidemiological declaration and had a set study protocol. For data collection and analysis, a wide variety of databases were consulted, including Google Scholar, Cochrane Library, Embase, OVID, and PubMed, in search of paper published between 1996 to 2020.
Data pooling
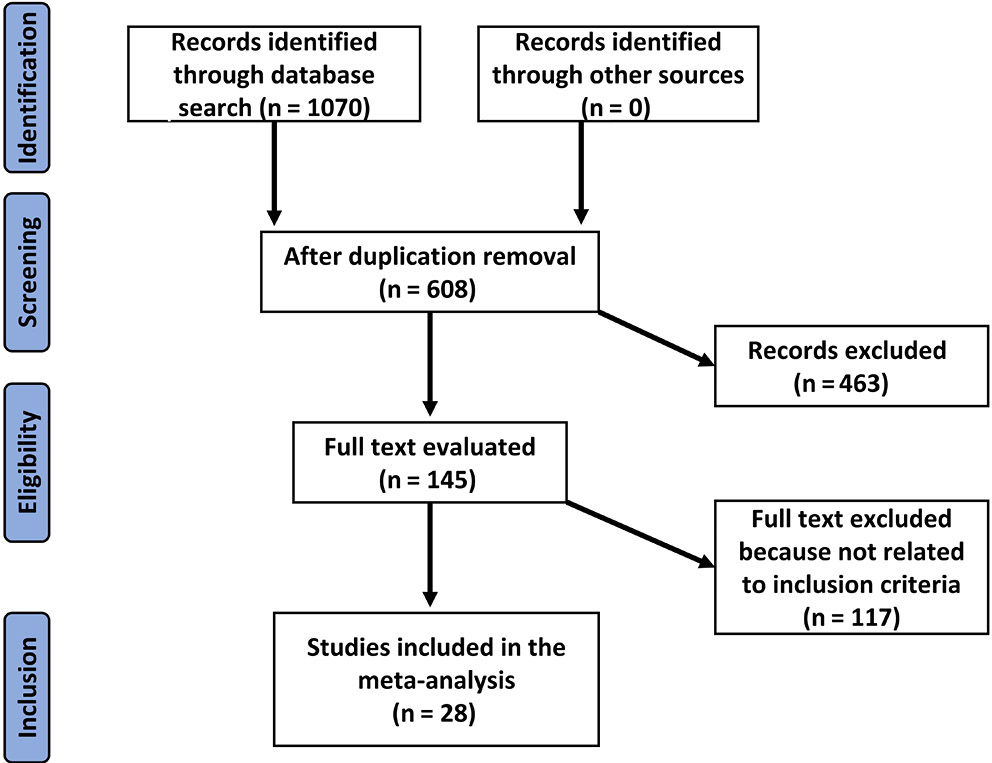
Evaluating the association between the occurrence of parkinsonism and alterations in the gut microbiota and pathogens is the subject of prospective and retrospective investigations. There was no language limitation for study inclusion; only human-related research was included regardless of the sample size. In the current meta-analysis, non-interventional studies such as reviews, editorials and research letters were excluded. Figure 1 depicts the comprehensive study identification procedure.
Eligibility and inclusion criteria
An evaluation of the association between the incidence of parkinsonism and alterations in the gut microbiota and infections was performed to compile this summary.
The study of sensitivity was limited to articles reporting the association between the occurrence of parkinsonism and alterations in the gut microbiota and pathogens. In addition to examining the impact of the occurrence of infections in PD patients compared to healthy controls, this study also examined the relationship between the two. Various subject types were compared to the interventional groups for subclass and sensitivity analyses.
For an article to be considered for inclusion in our meta-analysis, it had to satisfy the following inclusion criteria:
1. Only clinical trials involving humans were included.
2. Subjects with PD were the intervention population examined against a control group.
3. Intestinal microorganisms, including microbiota, discovered in stool samples, were the main point of research in recruited studies.
The exclusion criteria were:
1. Studies that did not evaluate the relationship between specific microorganisms found in the gut microbiota with the risk of PD.
2. Review articles, research letters, books, or book chapters.
Identification
Under the PICOS principle, the following protocol of search tactics was established: P (population) – subjects with PD; I (intervention/exposure) – stool samples for detection of intestinal bacteria; C (comparison) – quantity and incidence of gut microorganisms in PD compared to controls; O (outcome) – incidence levels of HP and intestinal microorganisms in PD against the control group; S (design of the study) – prospective or retrospective clinical studies.
Relevant articles published till August 2022 were searched through a comperhensive search of Cochrane Library, Embase, PubMed, OVID, and Google Scholar databases, using the key words and associated phrases given in Table 1. The titles and abstracts of all the articles that had been compiled in a reference management program were reviewed, and all studies that did not correlate postoperative results with rehabilitation strategies were excluded. The 2 authors (BL and YD) served as reviewers for the selection of appropriate studies.
Screening
According to the following criteria, data from different studies were collected to include: study- and subject-related features in a standard format; the surname of the first author; the length of the study; the year of publication; the country of the study; the design of the study; the population type recruited in the study; the total number of subjects; qualitative and quantitative evaluation method; demographic data; clinical and treatment characteristics; information source; outcome evaluation; and statistical analysis methods. Each study was assessed for bias, and the methodological quality of the chosen studies was evaluated by 2 of the authors in a blinded fashion.
Statistical analyses
Mean difference (MD) and 95% confidence interval (95% CI) were determined using a random effects model. Due to a substantial heterogeneity in certain groups and inconsistent technique in other groups, all groups were evaluated using the random model. Utilizing the fixed models required proof of high similarity between the included studies and low heterogeneity (I2) level. The I2 index was calculated as a percentage value ranging from 0% to 100%. The absence of heterogeneity was indicated by I2 of 0%. The I2 values ranged from >0% to <25%, 25% to <75% and ≥75% indicating low, moderate and high heterogeneity, respectively. As indicated previously, the subcategory analysis was performed by stratifying the first evaluation into result categories. The publication bias was analyzed statistically using the Begg’s test and the Egger’s tests, and it was deemed to exist when p < 0.05. The p-values were determined using a two-tailed test. Statistical analyses and graphs were produced using Jamovi 2.3 software (https://www.jamovi.org/download.html).
Results
After a review of 1070 relevant studies, 28 studies published between 1996 and 2019 were included in the meta-analysis because they fit the inclusion criteria.7, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 Table 2 (characteristics of included studies: year, country, number of subjects, and study quality) summarizes the findings of these investigations.
Small intestinal bacterial overgrowth
The analysis included 4 studies that showed significantly increased growth of small intestinal bacteria in PD subjects compared with controls (p < 0.001, MD = 1.66 (95% CI: 1.1623–2.15), I2 = 7.1%). Neither the rank correlation nor the regression test showed funnel plot asymmetry (p = 0.33 and p = 0.18, respectively) (Figure 2A).
Helicobacter pylori
The analysis comprised 11 studies that found a significantly higher incidence of HP among PD subjects compared with controls (p < 0.001, MD = 0.51 (95% CI: 0.27–0.74), I2 = 44.5%). Neither the rank correlation nor the regression test showed funnel plot asymmetry (p = 0.44 and p = 0.4, respectively) (Figure 2B).
Bifidobacteriaceae
The analysis included 8 studies that showed significant abundant levels of Bifidobacteriaceae in PD subjects compared with controls (p = 0.002, MD = 0.35 (95% CI: 0.09–0.61), I2 = 76%). Neither the rank correlation nor the regression test showed funnel plot asymmetry (p = 0.11 and p = 0.22, respectively) (Figure 3A).
Christensenellaceae
Five studies included in this analysis showed significantly higher levels of Christensenellaceae in PD subjects compared with controls (p = 0.003, MD = 0.20 (95% CI: 0.07–0.34), I2 = 0.67%). Neither the rank correlation nor the regression test revealed any funnel plot asymmetries (p = 0.81 and p = 0.85, respectively) (Figure 3B).
Faecalibacterium
Five studies included in this analysis showed significantly lower levels of Faecalibacterium in PD subjects compared with controls (p = 0.01, MD = −0.33 (95% CI: −0.65–−0.02), I2 = 72.2%). Neither the rank correlation nor the regression test indicated any asymmetry in the funnel plot (p = 1.00 and p = 0.52, respectively) (Figure 3C).
Lachnospiraceae
The analysis of 7 studies that met the inclusion criteria showed significantly lower levels of Lachnospiraceae in PD subjects compared with controls (p = 0.005, MD = −0.34 (95% CI: −0.58–−0.1), I2 = 62.7%). No significant asymmetry was found using either the rank correlation or the regression test (p = 0.77 and p = 0.44, respectively) (Figure 3D).
Prevotellaceae
Nine studies included in this analysis showed significantly lower levels of Prevotellaceae in PD subjects compared with controls (p < 0.001, MD = −0.37 (95% CI: −0.62–−0.11), I2 = 72.4%). There was no significant bias according to the rank correlation or the regression test (p = 0.92 and p = 0.85, respectively) (Figure 3E).
Ruminococcaceae
The analysis included 10 studies that showed a nonsignificant difference between PD subjects and controls (p = 0.1, MD = 0.66 (95% CI: −0.19–1.51), I2 = 96.8%) regarding Ruminococcaceae levels. The regression test revealed a significant publication bias (p = 0.001), although the rank correlation test did not (p = 0.12) (Figure 3F).
Verrucomicrobiaceae
The analysis included 7 studies using the random effects model that showed significant levels of Verrucomicrobiaceae (p < 0.001, MD = 0.45 (95% CI: 0.21–0.69), I2 = 67.9%). Neither rank correlation nor regression test suggested a publication bias (p = 1.00 and p = 0.6279, respectively) (Figure 3G).
Discussion
Patients with PD were found to have significantly lower levels of numerous metabolic products secreted by their gut bacteria, which may lead to constipation.43 Functional differences between PD patients and controls were found in the glucuronate and tryptophan degradation pathways.19 Several bioactive molecules with putative neuroprotective effects, including short-chain fatty acids (SCFAs), ubiquinone and salicylate, were modified in PD, as were the substances associated with neurodegeneration, including ceramides, sphingosine and trimethylamine N-oxide.44 Disease duration, motor symptom severity and non-motor symptom severity were all observed to correspond with several gut bacteria.45 Changes in the composition of the metabolome, such as the decrease in SCFAs, have also been linked to impairments in cognition. In PD, decreased butyrate levels have been linked to increased levels of postural instability and gait disorder.44 Total counts of gut microbiota declined over the course of PD progression and differed between worsening and stable PD groups, according to a 2-year follow-up study that suggested they could be utilized as a diagnostic tool for monitoring the course of PD.46, 47 The levels of Bifidobacteriaceae were found to be lower in the severe case of PD compared with the stable case and they were linked to the delusional symptoms and hallucination.47 These manifestations could be a result of a lower antioxidant effect associated with a lower Bifidobacteriaceae count.46 In the current study, the level of Bifidobacteriaceae was compared between PD subjects and healthy controls, and was higher in PD subjects. Some studies indicated a lower abundance of Bifidobacteriaceae, while the majority indicated a higher abundance.48
Twenty-five genetic markers from the gut microbiota were found to be significantly altered in PD, and an index was developed from these changes; this index could differentiate between individuals with PD and those with multiple system atrophy.45 Heterogeneous reactions to levodopa, such as diminished efficacy and unpleasant side effects, are reported among patients with PD. This may be due, in part, to variations in the gut microbial activity.49
Delivery method, newborn feeding, nutrition, lifestyle, culture, geography, age, gender, and other factors all contribute to the unique composition of each person’s gut microbiota.50 However, even when we controlled for these variables, we still discovered that the quantity of Prevotellaceae, Faecalibacterium, Lachnospiraceae, Bifidobacteriaceae, Verrucomicrobiaceae, and Christensenellaceae all varied in a PD patient’s gut, regardless of where the study was conducted. These shifts in gut microbiota were mostly associated with PD clinical markers or may have operated as PD progression promoters. It is now well established that inflammation contributes to the onset of PD, activating microglia that play a critical role in the destruction of dopaminergic neurons and the accumulation of α-synuclein.51 Short-chain fatty acids may have neuroprotective effects due to their anti-inflammatory and antioxidant properties, which could aid in the regulation of neuroinflammation and gut permeability.52 Microglial activation and an uptick in the risk of α-synuclein deposition in PD have been linked to an imbalance in the bacteria that produce SCFAs. In terms of the lipid metabolism pathway, oxidative stress and inflammatory reaction may be facilitated by lipid dysregulation, hence contributing to PD pathophysiological process.53 In synucleinopathy, lipids influence α-synuclein aggregation and transit.53, 54 This suggests that alterations in the gut microbiota that have a role in lipid metabolism may also contribute to the pathophysiology of PD. A previous review showed results similar to the current study, expressed as the higher abundance of Verrucomicrobiaceae, Christensenellaceae and Bifidobacteriaceae in PD subjects compared with controls.55 In addition, it was reported that the abundance of Prevotellaceae is lower in PD subjects compared with controls. While the mentioned review showed different results regarding Ruminococcaceae which have been reported to be highly abundant in PD subjects in 3 studies,55 in the current research, 10 studies have been selected and analyzed to reflect the conclusion of a non-significant difference between PD and controls. Another study by Romano et al. showed the results similar to the current studies regarding the abundance of Bifidobacteriaceae, Lachnospiraceae and Faecalibacterium in PD subjects compared with controls.56
Various hypotheses have been proposed in order to to explain the mechanism(s) connecting HP and eventual PD. For example, endotoxins from Gram-negative bacteria may cause microglia to produce inflammatory markers via the humoral or vagal afferent pathways, leading to the notion of microglia-mediated neuroinflammation. Nitric oxide can be transferred via the vagal pathway57 and lead to α-synuclein misfolding,58, 59 and interleukin (IL)-1 and tumor necrosis factor alpha (TNF-α) can pass the blood–brain barrier and harm the dopaminergic neurons via the humoral pathway.21 The α-synuclein is thought to be a prion-like protein that is improperly deposited in the ENS of PD patients and may acquire the access to the central nervous system via the vagus nerve.60, 61 In its soluble state, the protein would be able to pass the blood–brain barrier.62 It has also been suggested that apoptosis may play a role in the link between HP infection and eventual PD,63 with the infection entering the brain via the oral–nasal olfactory route, activating apoptosis via the mitochondrial apoptotic pathway and causing dopaminergic neuron degeneration. The HP infection may promote α-synuclein-mediated neuroinflammation64 by disrupting gut microbiota and host homeostasis,64 possibly via effects on microbial SCFA signaling and microglial activation. Furthermore, small intestinal bacterial overgrowth may perform an alternate or synergistic role to HP, activating degenerative processes in the ENS.28 Bacterial overgrowth in the small intestine has been linked to an increased risk for developing PD28, 65 and has been shown to predict motor impairment in PD on its own. Increased levels of Enterococcus spp. and Staphylococcus aureus were observed in the stomach and duodenum in other investigations evaluating the impact of HP infection on the digestive tract.66 Increased levels of E. coli and Enterococci were also found in the cecum, and Bacteroides/Prevotella spp. were found in the colon, according to another study.67
Limitations
Many studies discusing a similar topic were excluded as they did not meet the inclusion criteria. Furthermore, the influence of race on the shown outcomes was not assessed in all of the included research. In addition, patients with GI symptoms were most commonly diagnosed using endoscopic mucosal biopsy due to the limited funding for 13C urea breath testing. This may suggest that the HP infection had been prevalent for some time. Similar difficulties were encountered when attempting to differentiate between HP infection and gastritis or peptic ulcers.
Conclusions
Parkinson’s subjects showed a higher degree of alteration of gut microbiota and pathogens compared with healthy subjects. Higher incidence of small intestinal bacteria overgrowth and HP were significantly related to parkinsonism. Future multicenter randomized trials are still needed.