Abstract
Background. Endometriosis is a chronic inflammatory pathology that can cause persistent pelvic pain and infertility by affecting women of reproductive age. It is defined as the placement of endometrial tissue outside the uterine cavity. Hormonal, genetic and immunological factors have an effect on the development of endometriotic implants. Adalimumab is a monoclonal antibody specific for tumor necrosis factor alpha (TNF-α), used in the treatment of autoimmune diseases.
Objectives. To investigate the effectiveness of adalimumab on histopathological and biochemical values in rats with experimental endometriosis.
Materials and methods. This study is a comparative, prospective, experimental rat study. Wistar albino female rats were divided into 4 groups. Group 1 was separated as the control group. Endometriotic implants were simultaneously induced in group 2 and group 3. After 4 weeks, developing endometriotic foci were measured. Adalimumab (5 mg/kg) was simultaneously intraperitoneally (ip.) administered to group 3 and group 4 for 4 weeks. At the end of the study, histopathological scoring and fibrillin-1 scoring were performed. Total antioxidant status (TAS), total oxidant status (TOS) and malondialdehyde (MDA) values were measured. Findings in all groups were compared.
Results. When group 1 and group 2 were compared, the histopathological score, as well as MDA and TOS levels increased, while TAS levels decreased in group 2 (p < 0.001). After adalimumab treatment, the average endometriotic implant size in group 3 (0.32 ±0.002 mm) decreased compared to group 2 (0.77 ±0.04 mm) (p = 0.032). While fibrillin-1 score increased in group 2 and group 3 compared to group 1, it decreased in group 3 compared to group 2 (p < 0.001). Histopathological score decreased, TAS levels increased and MDA levels decreased in group 3 compared to group 2 (p < 0.001).
Conclusions. Adalimumab may play a role in the regression of endometrial implants by showing antioxidant and anti-inflammatory effects on histopathological damage and fibrosis.
Key words: endometriosis, rat, adalimumab, antioxidant effect, fibrillin-1
Background
Endometriosis is a common gynecological pathology affecting 5–10% of women in the reproductive age.1 It causes symptoms such as chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility.2 It has negative effects on ovarian reserve, tubal anatomy, embryo quality, and implantation.3 The pathophysiology of endometriosis is still not entirely clear and several theories have been proposed. It has been stated that proinflammatory cytokines play a role in the pathophysiology of endometriosis.4, 5, 6 There are cytokines responsible for inflammatory reactions and tissue neovascularization. Interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α) have been previously studied in the pathogenesis of endometriosis.7 The estrogenic microenvironment activates peritoneal macrophages with secretion of TNF-α and IL-1, which are pro-inflammatory cytokines. The increase in the level of IL-6 and TNF-α in the peritoneal fluid of patients with abnormal immune cell activity shows the role of cytokines in the pathogenesis.8, 9, 10
Endometriosis is a pathology caused by the abnormal proliferation of endometrial tissue outside the uterine cavity.1 Transforming growth factor beta (TGF-β) plays a key role in the pathological growth of many fibrotic tissues.11 The TGF-β stimulates the expression of extracellular matrix proteins.12, 13 Its receptors have been detected in leiomyomas and myometrium, and it has been shown that estrogen and TGF-β expression are increased in endometriosis, and TGF-β activity mediates the effects of estrogen.14 This interaction may play a role in the development of endometriosis. Fibrillin-1 is a protein that indicates the activation of TGF-β.15
Adalimumab is a fully human immunoglobulin G (IgG)1 neutralizing monoclonal antibody specific for TNF-α.16, 17 It is used in the treatment of many autoimmune diseases, such as rheumatoid arthritis, Crohn’s disease and psoriatic arthritis.16 In previous experimental studies in rats, agents such as etanercept and infliximab have been reported to decrease TNF-α levels in peritoneal fluid in rats with endometriosis.18, 19 As far as we know, there is no study reporting the relationship between histopathological and biochemical changes and adalimumab in endometriosis.
Objectives
The aim of this study is to determine whether adalimumab can be an effective medical treatment agent, by examining its histopathological and biochemical effects on endometriosis.
Materials and methods
The experiments in this study were carried out in accordance with the National Institutes of Health (NIH) animal research guidelines and were approved by Adıyaman Training and Research Hospital Ethics Commitee (approval No. 2019/062, December 26, 2019).
Animals and experimental protocol
Twenty-eight Wistar albino female rats, 10–12-week old, weighing 250–280 g, were divided into 4 groups with 7 animals in each group. No procedures were performed for 7 days to ensure the adaptation of animals. Rats were housed at 20 ±22°C room temperature during the adaptation and experimental period, in rooms with 12-hour light and 12-hour dark light cycle, with food and water ad libitum. The animals were classified into 4 groups (control group, endometriosis group, endometriosis + adalimumab group, and adalimumab group).
All rats were anesthetized by intramuscular administration of 60 mg/kg ketamine hydrochloric acid (Ketalar; Warner-Lambert, Istanbul, Turkey) and 7 mg/kg xylazine hydrochloric acid (Rompun; Bayer, Istanbul, Turkey).
In group 1 (control group, n = 7), the pelvic region was opened with laparotomy and the adnexa were localized as the right and left adnexa. After the adnexa was localized with the right and left uterine horn, the abdominal wall was closed with 4-0 nylon sutures. No action was taken until the end of the experiment.
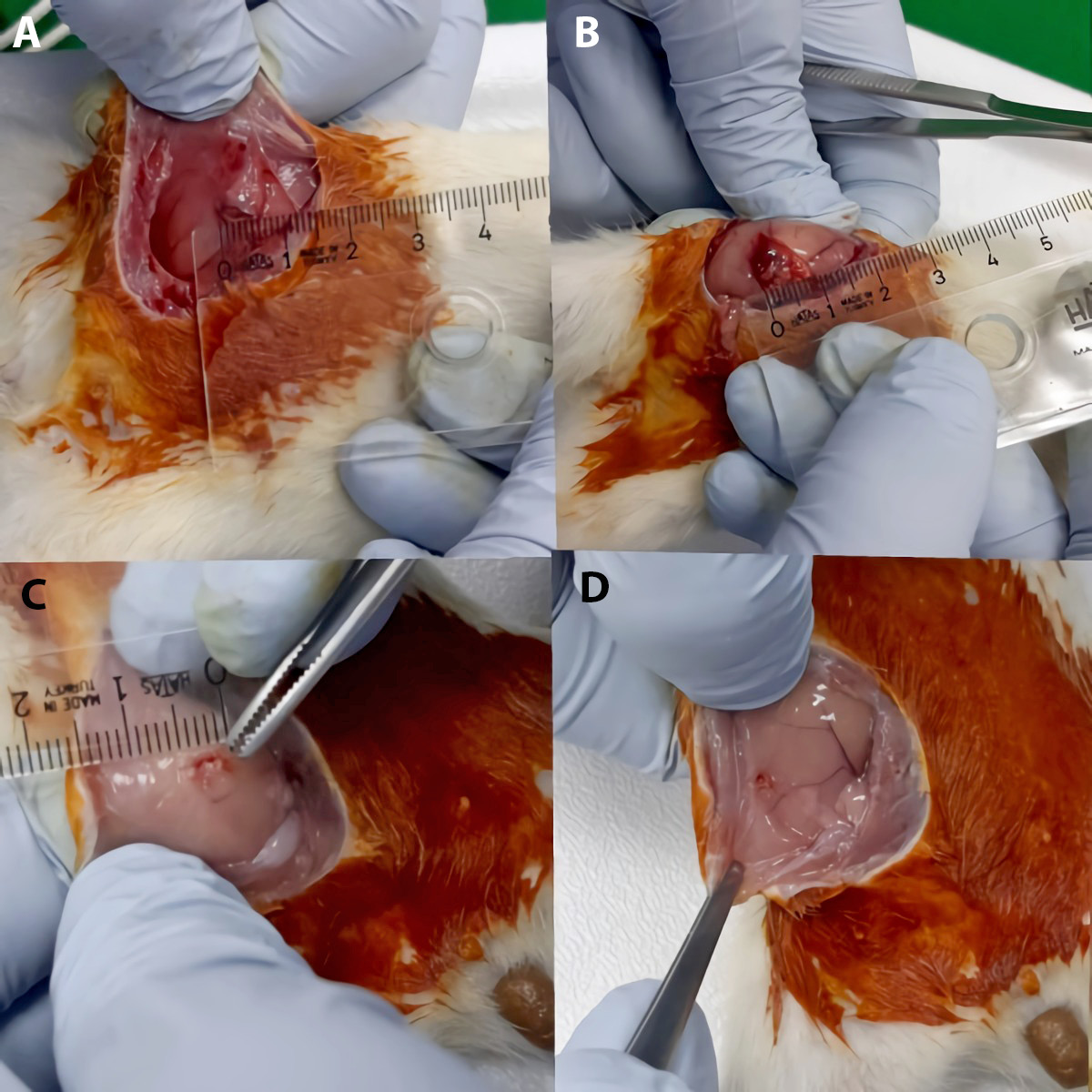
In group 2 (endometriosis group, n = 7), the autotransplantation method was used for the induction of endometriosis. The reproductive cycles of the animals were controlled by vaginal smear and the rats in the estrus phase were selected. After general anesthesia, a vertical incision was made to expose the uterus. Both uterine horns were removed from the cervix to the point located about 1 cm from the ovaries. Electrocoagulation was used for hemostasis. The uterine horns were divided longitudinally, exposing the endometrium. Without removing the myometrium, endometrial segment (5 × 5 mm) was implanted into the peritoneal surface of the right abdominal wall, so that the endometrium came into contact with the peritoneal surface. Both ends of the implants were fixed to the interior with 6-0 nonabsorbable polypropylene suture.20 All rats were allowed to recover for 4 weeks following surgical induction of endometriosis. At the end of the 4 weeks, the rats were operated to observe the growth of endometriotic implants. The equation: 6 × length × width × height of the implant was used to measure the surface areas of the implants and calculate the endometriotic volume. After the endometriotic lesions were photographed, the size of the lesion was recorded and the peritoneal cavity was closed. No procedure was performed on rats for 4 weeks after the development of endometriosis.
In group 3 (endometriosis + adalimumab group, n = 7), after the reproductive cycles of the animals were controlled by vaginal smear and the rats in the estrous phase were selected, the endometriosis induction was performed using autotransplantation method. At the end of the four-week period, after calculating the endometriotic volume and photographing the lesions, 5 mg/kg adalimumab was administered intraperitoneally (ip.) for 4 weeks.21
In group 4 (adalimumab group, n = 7), after the adnexa was localized with the right and left uterine horn, the abdominal wall was closed with 4-0 nylon sutures and 5 mg/kg adalimumab was administered ip. every day for 4 weeks, to start at the same time as group 2.
Histopathological evaluation
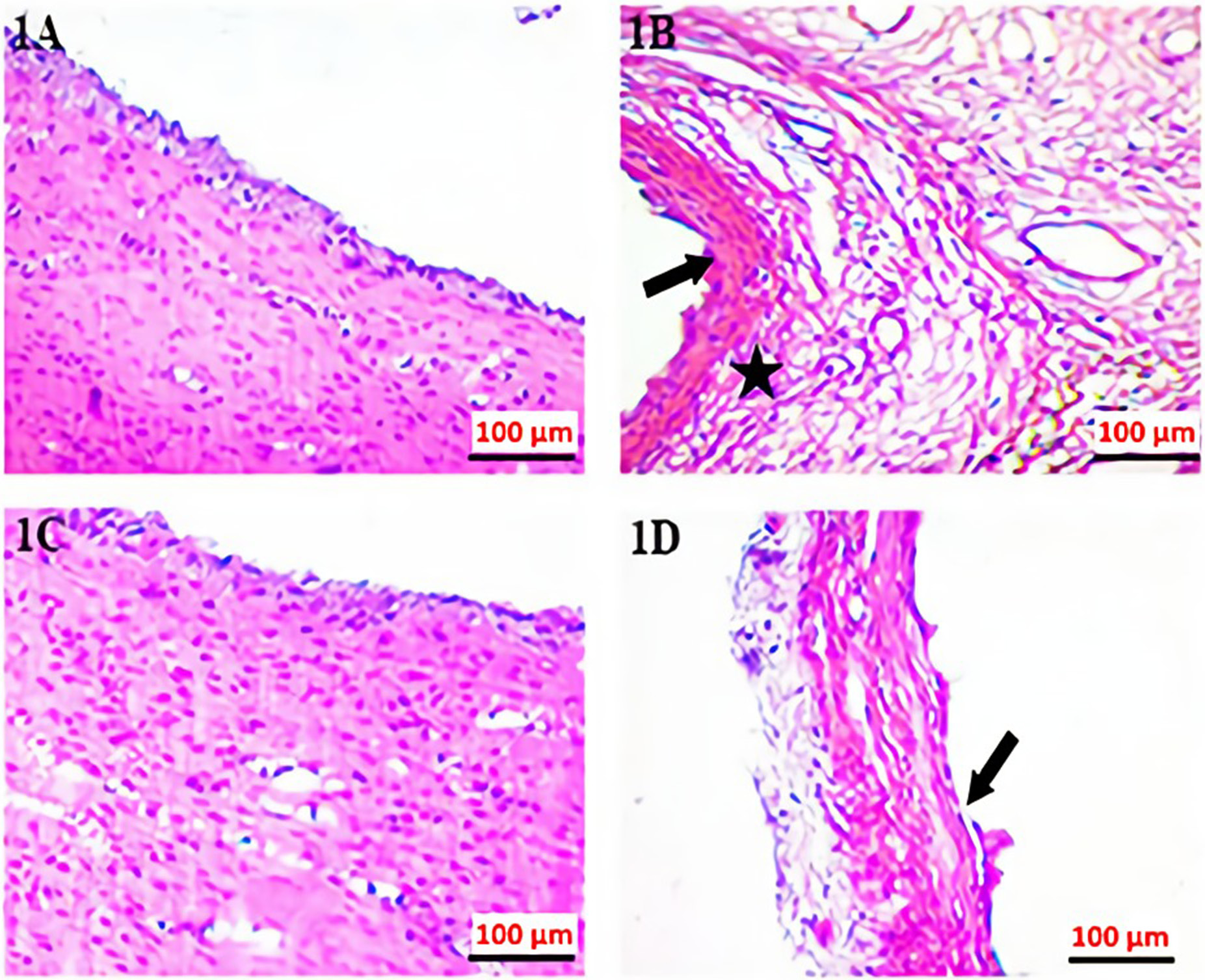
The excised endometrial implants were fixed with 10% formalin solution upon histopathological examination. Sections approx. 5-μm thick were taken from a formalin-fixed endometriotic implant. Samples were stained with hematoxylin and eosin (H&E) and examined under light microscopy. Histopathological scoring was done22 based on a following rating scale: +3: a well-preserved epithelial layer; +2: a moderately-preserved epithelial layer (leukocyte infiltrated epithelium); +1: a poorly-preserved epithelial layer (sparse epithelial cell); 0: no epithelial cells.
Immunohistochemical examination
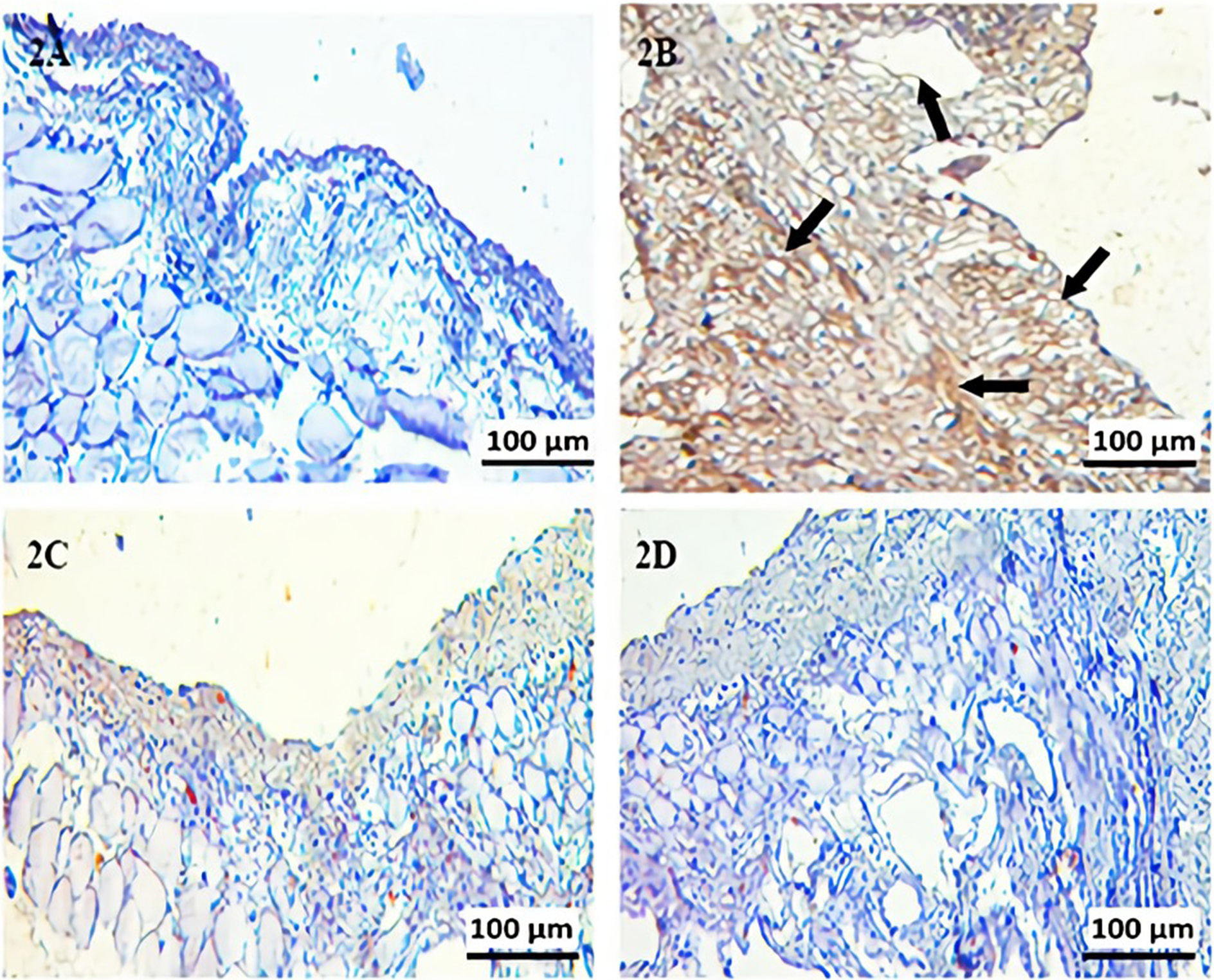
For fibrillin-1 antigen retrieval, sections were rehydrated, then boiled in a microwave oven (750 W) 7 times for 5 min each in citrate buffer solution (pH 6). Sections were cooled at room temperature for 20 min, washed 3 times for 5 min each with phosphate-buffered saline (PBS; P4417; Sigma Chemical Co., St. Louis, USA), then incubated for 5 min with hydrogen peroxide block solution (TA-125-HP; Lab Vision Corp., San Francisco, USA) to block endogenous peroxidase activity. Then, the sections were washed 3 times for 5 min each with PBS. After applying Ultra V Block (TA-125-UB; Lab Vision Corp.) for 5 min, sections were incubated with primary antibodies for fibrillin-1 (rabbit polyclonal bs-1157R; Bioss Antibodies, Woburn, USA) and diluted 1:200 at room temperature for 60 min in a humid environment. After being washed with PBS 3 times for 5 min each, the sections were incubated at room temperature for 30 min in a humid environment with secondary antibody (biotinylated goat anti-mouse/rabbit IgG, TP-125-BN; Lab Vision Corp.). Sections were washed with PBS 3 times for 5 min each and incubated at room temperature for 30 min in a humid environment with streptavidin peroxidase (TS-125-HR; LabVision Corp.) and then placed in PBS. The 3-amino-9-ethylcarbazole (AEC) substrate + AEC chromogen (AEC substrate, TA-015 and HAS, AEC chromogen, TA-002-HAC; Lab Vision Corp.) solution was dripped on the sections. The sections were washed with PBS. Sections were counterstained with Mayer’s hematoxylin, passed through PBS and distilled water and mounted with Large Volume Vision Mount (TA-125-UG; Lab Vision Corp.). Sections were evaluated and photographed using a digital microscope camera (Leica DFC295; Leica Camera AG, Wetzlar, Germany). The histoscore, which reflects the prevalence of immunoreactivity of fibrillin-1 on the tissue, was based on the rating scale: 0.1: < 25%; 0.4: 26−50%; 0.6: 51−75%; 0.9: 76−100%, and intensity of immunoreactivity: 0: unstained; 0.5: little staining; 1: some staining; 2: moderate staining; 3: strong staining. The histoscore was measured using the following equation:
Determination
of malondialdehyde (MDA) level
Determination of malondialdehyde level was performed by applying the Esterbauer method, which is a lipid peroxidation measurement method.23 Malondialdehyde reacting with thiobarbituric acid at 90–95°C forms pink-colored chromogen. Fifteen minutes later, the absorbances of the rapidly cooled samples were read spectrophotometrically at 532 nm. The results are expressed in nmol/g.
Determination of total antioxidant status (TAS) and total oxidant status (TOS) levels
Total antioxidant status and total oxidant status were measured in serum samples using enzyme-linked immunoassay (ELISA) method. The TAS (Rat TAS Catalog No. YLA3889Ra; YL Biotechnology Co., Ltd, Shanghai, China) and TOS (Rat TOS Catalog No. YLA1892Ra; YL Biotechnology Co., Ltd) levels were measured in accordance with the procedures specified in the catalog of kits. The measurement range of the Rat TAS ELISA kit was: 1–300 pg/mL, intra-assay coefficient of variation (CV) <10%, inter-assay CV < 12%, sensitivity 0.54 pg/mL. The measurement range of the Rat TOS ELISA kit was 0.02–60 U/mL, intra-assay CV < 10%, inter-assay CV < 12%, sensitivity 0.013 U/mL. The automatic washer BioTek ELx50 (BioTek Instruments, Winooski, USA) was used for plate washing, while ChroMate Microplate Reader P4300 devices (Awareness Technology, Palm City, USA) were used for absorbance readings. The unit of test results is specified for serum samples in U/mL.
Statistical analyses
The IBM SPSS v. 22 software (IBM Corp., Armonk, USA) program was used to analyze the data. The Shapiro–Wilk test was used as a normal distribution test. The Shapiro–Wilk test results for MDA measurement were reported as p = 0.830 for group 1, p = 0.898 for group 2, p = 0.881 for group 3, and p = 0.716 for group 4. The Shapiro–Wilk test results for TAS measurement were 0.274 for group 1, 0.540 for group 2, 0.648 for group 3, and 0.355 for group 4. The Shapiro–Wilk test results for TOS measurement were reported as p = 0.707 for group 1, p = 0.598 for group 2, p = 0.700 for group 3, and p = 0.944 for group 4. The Shapiro–Wilk test results for fibrillin-1 measurement were reported as p = 0.812 for group 1, p = 0.652 for group 2, p = 0.717 for group 3, and p = 0.941 for group 4. Levene’s homogeneity test was performed, in which the data met the assumption of normal distribution for each group, and the results were p = 0.170 for MDA, p = 0.050 for TAS, p = 0.654 for TOS, and p = 0.191 for fibrillin-1 measurement. It was observed that the variances were homogeneous. One-way analysis of variance (ANOVA) test (post hoc Bonferroni test) was used in the analysis of the data conforming to the normal distribution. The score variable does not show a normal distribution. The p-value of the score variable is <0.001. Spearman’s correlation test was used for the values where the score did not show normal distribution, and Pearson’s correlation test was used for the others. The value of p < 0.05 was considered statistically significant.
Results
Histopathological scoring
The H&E staining and immunohistochemistry staining histopathological images were shown in Figure 1 and Figure 2. There was a statistically significant difference between the measurements in different groups. A statistical difference was observed between group 2 and group 3 (p < 0.001) (Table 1). When group 1 and group 2 were compared, the increase in histopathological damage in group 2 was statistically significant (p < 0.001). When group 3 and group 2 were compared, the histopathological damage score was significantly decreased in group 3 (p < 0.001).
Macroscopic examination
The mean size of rats endometriotic implants in group 2 was 0.77 ±0.04 mm2. After adalimumab treatment, it measured 0.32 ±0.002 mm2. This decrease was statistically significant (p = 0.032) (Figure 3).
Biochemical analysis
Post hoc one-way ANOVA results for biochemical measurements are given in Table 2.
MDA level
The MDA levels significantly increased in group 2 compared with group 1 (p < 0.001). The MDA values were significantly decreased in group 3 compared with group 2 (p < 0.001). There was no statistically significant difference between group 1 and group 4 (p > 0.05) (Table 1, Table 2).
TAS level
The TAS levels were significantly decreased in group 2 compared with group 1 (p < 0.001). The TAS values increased in group 3 compared to group 2 (p < 0.001). There was no statistically significant difference in TAS values between group 1 and group 4 (p > 0.05) (Table 1, Table 2).
TOS level
The distribution of scores in respective groups is given in Table 3. The TOS levels were significantly increased in group 2 compared with group 1 (p < 0.001). The TOS values in group 2 were significantly decreased compared with group 4 (p < 0.001). However, there was no statistical difference in TOS values between group 2 and group 3 (p > 0.05). The TOS values were significantly increased in group 4 compared with group 1 (p = 0.016) (Table 1, Table 2).
When histopathological scores and biochemical values were compared, there was a strong negative correlation between MDA value and TOS value and a strong positive correlation between MDA value and TAS value (p < 0.001) (Table 3).
Immunohistochemistry examination score
In the immunohistochemistry examination, fibrillin-1 scores differed significantly for 4 groups. According to the post hoc one-way ANOVA analysis between groups, fibrillin-1 activity increased in groups 2 and 3 compared to group 1 (p < 0.001). Moreover, fibrillin-1 immune reactivity decreased in group 3 compared to group 2 (p < 0.001) (Table 3).
Discussion
In this study, the effect of adalimumab on histopathological changes in endometriotic implants and its antioxidant effects were investigated. The size of endometriotic implants decreased after adalimumab treatment, as shown with macroscopic examination. Histopathological damage score increased in rats with experimental endometriosis and decreased with adalimumab administration. Fibrillin-1 score was increased in endometriotic implants, but fibrillin-1 score decreased in endometriotic implants treated with adalimumab. In addition, adalimumab decreased MDA levels and increased TAS levels in endometriotic implants. These findings show the histopathological improvement and antioxidant activity caused by adalimumab treatment in endometriotic implants.
Oxidative stress and inflammation play an important role in the pathogenesis of endometriosis.24, 25, 26 Moreover, studies have shown that erythrocytes, apoptotic endometrial tissue, cell debris in the peritoneal cavity, and macrophages induce oxidative stress and inflammation, and cause endometriosis.26 It is known that the balance between reactive oxygen species (ROS) and antioxidants is lost in the development of endometriosis.27 The presence of ROS in the environment affects gene expression, causing protein dysfunction and cellular damage.26, 27, 28 This oxidative stress can be both a cause and a consequence of endometriosis.26 In addition, the role of mitophagy and autophagy in the pathophysiology of endometriosis supports the role of oxidative damage.28 It has been hypothesized that eliminating oxidative stress may reduce the histopathological grade in endometrial implants and many studies have been conducted on this subject.27, 29, 30
Proinflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α have also been shown to play a role in the pathology of endometriosis.31 It has been demonstrated that there is an increase in TNF-α levels in the peritoneal fluids of women with endometriosis.32 It is thought that endometrial cell proliferation increases and endometriotic lesions develop by inducing IL-8 secretion with the increase of TNF-α.33, 34 In previous studies using etanercept, which has an anti-TNF-α activity, it was reported that endometriotic implants were reduced and histopathological scores decreased.35 In another study, etanercept was reported to decrease MDA levels in endometriotic focus.36 In our study, adalimumab treatment has decreased endometriotic implant dimensions, histopathological damage score and fibrillin-1 score, which is a fibrosis marker.
Fibrillin-1 has been associated with heart and liver fibrosis, and has been shown to cause the expression of extracellular matrix proteins in various studies in rats.37, 38 In addition, it has been previously observed that topical application of estrogen increases the activity of fibrillin-1 and other extracellular matrix proteins.39 Moreover, it was demonstrated that fibrillin-1 activity increased in proportion to the size in leiomyomas.40 In our study, the increase in fibrillin-1 score in endometriotic implants may be related to the role of the estrogenic microenvironment in endometrial implant development. Since estrogen levels were not measured in our study, large studies can be conducted to investigate this hypothesis.
Adalimumab is a drug that is effective both as monotherapy and in combination with disease-modifying antirheumatic drugs in the treatment of many chronic, inflammatory, immune-mediated diseases.16 It has been reported that adalimumab decreases cell proliferation and increases the function of natural immune pathways by decreased IL-8 levels.41 Considering the role of proinflammatory cytokines in the pathogenesis of endometriosis, it is not surprising that endometriotic implants and histopathological score decreased along with adalimumab treatment. Moreover, the immunomodulatory activity of adalimumab may demonstrate decreased fibrillin-1 activation in endometriotic implants.
Limitations
This study has some limitations. The fibrillin-1 score is established primarily with immunohistochemistry staining, but there were no polymerase chain reaction (PCR) tests measuring the fibrillin-1 protein expressions. Second, the study was experimentally performed in rats. In order to generalize the results to society, studies should be carried out primarily with large human populations.
Conclusions
Adalimumab, through its antioxidant and anti-inflammatory effects, plays an important role in the treatment of histopathological damage and fibrosis in endometriotic implants developed experimentally. It can be used as a nonhormonal agent in the treatment of endometriotic implants. However, experiments with different parameters are needed in large groups of animals and humans.