Abstract
Natural products play significant roles in the development of novel drugs. One of such compounds is vanillin – a natural substance commonly used in food. Anticancer potential of the substance is continually encouraging researchers to conduct further investigations. A rising number of publications describe the role of 4-hydroxy-3-methoxybenzaldehyde (vanillin) in the process of inhibiting tumor growth. Four vanilloid receptors play significant roles in the response of cancer cells to the natural compound. Each of these proteins can be individually affected by vanillin; thus, the substance either leads to inhibition of the cell proliferation or increases the Ca2+ level. The TRPV1, a non-selective cation channel permeable to calcium, acts on cancer development and progression. Thus, vanilloid receptors have the potential to become the target for therapeutical research. Moreover, selective inhibitors of the receptor have proved their efficacy in vitro. CK2α is an antiapoptotic, cancer-sustaining protein and, therefore, the inhibitor of apoptosis. Thus, drugs that exhibit allosteric and ATP-competitive inhibition of the protein might be crucial for cancer therapy. CAMK4 is a protein kinase expression associated with a wide array of cancers. Also, MARK4 is another kinase responsible for the stability of microtubules, overexpressed in many cancer types. Studies concerning this protein revealed that microtubule impairment might be a cancer therapy direction.
This review aims to demonstrate the crucial role of described vanilloid receptors in inhibiting the proliferation of cancer cells and to prove the usefulness of using vanillin and its derivatives in the process of drug design.
Key words: vanillin, cancer, TRPV1 protein, CK2α protein, CAMK4 protein
Introduction
Vanilla extract consists of various natural substances. One of its most prominent components is vanillin (C8H8O3), a hydrophilic and phenolic aldehyde. The chemical structure of vanillin is comprised of aldehyde, hydroxyl and ether groups located around an aromatic ring. This compound is mostly known for its aromatic flavor and specific odor.1 Vanillin can be of both synthetic and natural origin. When extracted from the seedpods of Vanilla planifolia, the compound remains conjugated with β-D-glucose and initially lacks its taste properties. However, in the course of production, free vanillin is released from the glucoside by hydrolytic enzymes.2 Vanillin may be synthesized from small molecule natural compounds. Clove oil is the source of eugenol, which, through the oxidation of a vinyl group (attached to the aromatic ring), forms vanillin. In a similar way, vanillin may be synthesized from coniferyl alcohol (from spruce tree lignin) and from ferrulic acid (from rice). Interestingly, the vanillin precursor guaiacol may be found in petroleum.2 Due to its relatively simple structure, vanillin-like fragments are likely to be found in higher mass molecules. Following metabolism, radiation, heat and decomposition, vanillin may be released. For instance, irradiation of curcumin solution leads to the generation of free vanillin, feruloylmethane and acetone. In addition, vanillin remains one of the products of metabolism of curcumin.3
Vanillin has a significant antitumor potential,4 and its activity may be more valuable than previously considered.5 The antimutagenic effects of 4-hydroxy-3-methoxybenzaldehyde seem to be due to its influence on cell redox homeostasis and DNA repair pathways. However, not only does vanillin exhibit antimutagenic properties, but it is also considered to be an antimutagen.6, 7, 8 Moreover, it has been shown to have antioxidant,9, 10 antimicrobial,11 analgesic,12, 13 and anti-erythrocyte-sickling14 properties. Taking the quintessential properties of the discussed phenolic aldehyde into consideration, the process by which the described organic compound affects certain cells is worth further discussion and warrants closer analysis.
Vanillin receptors overview and their role in carcinogenesis
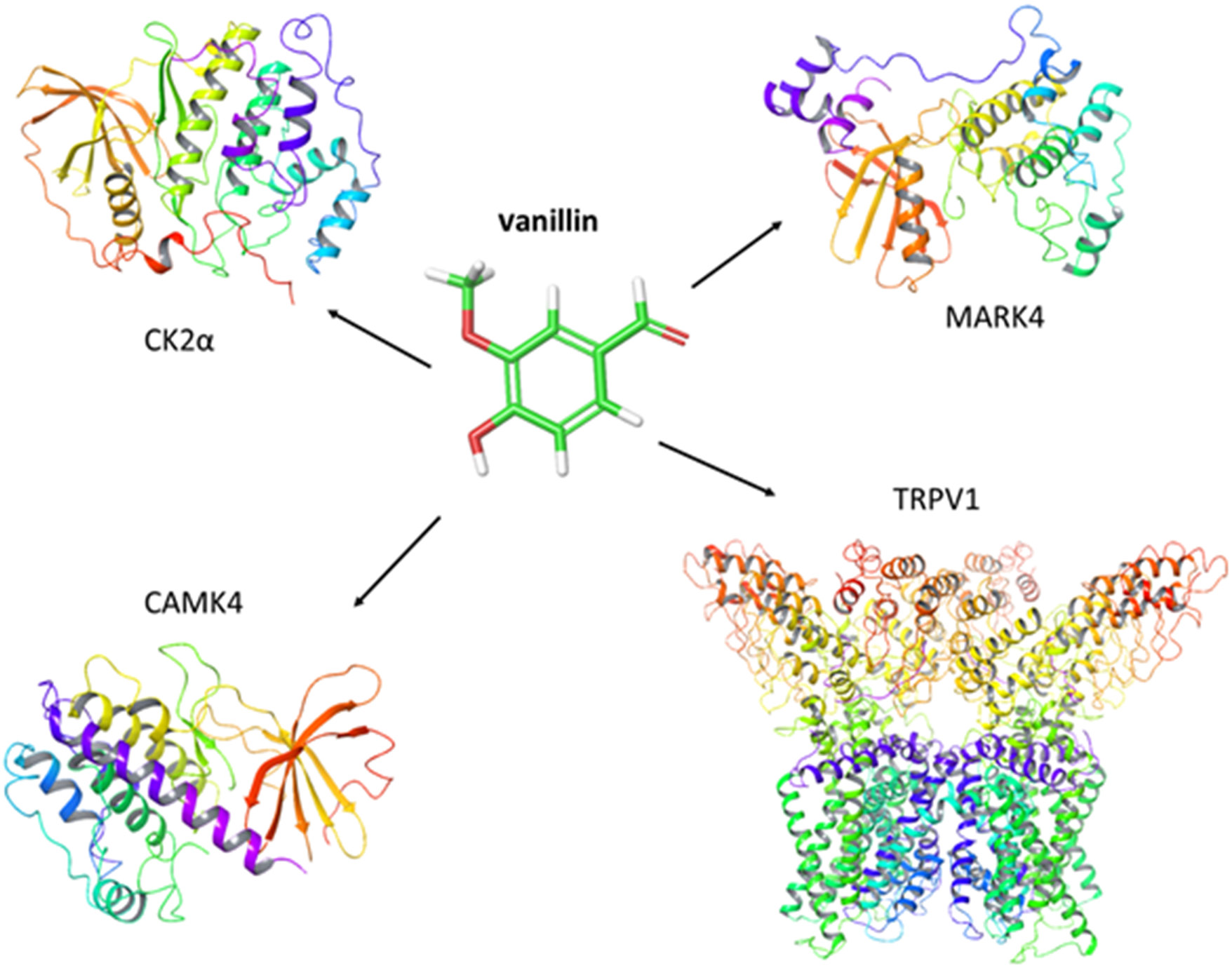
Vanillin may exert its antitumor potential by targeting membrane and intracellular receptors. The current literature suggests that this compound acts on 4 main proteins (transmembrane channel TRPV1) and 3 cytoplasmic peptides – MARK4, CAMK4 and CK2 (Figure 1).
TRPV1
The TRPV1 receptor belongs to the transient receptor potential gene superfamily that includes 28 separate genes, grouped into 6 subfamilies: TRPC, TRPV, TRPP, TRPM, TRPA, and TRPML.15, 16, 17 Each of these genes encode a non-selective cation channel responsible for a variety of functions in the organism.15, 16 These channels function as a molecular gateway, which transforms stimuli of both chemical and physical origins into action potentials. Moreover, these channels are the major transducers for a multitude of biological functions, including vision, taste, olfaction, mechanonsensation, osmosensation, and nociception.16, 18, 19 The products encoded by transient receptor potential (TRP) superfamily genes are crucially important sensory receptors, and among the members of the family, there is a group of thermoreceptors,20 which detect hot (TRPV1 and TRPV2) and cold (TRPA1) temperatures – from adverse stimuli to harmless ones (TRPV3, TRPV4 and TRPM8).15, 20 However, these channels are activated not only by temperature stimuli, but also by chemical substances like menthol (TRPM8), camphor (TRPV3), mustard oil (TRPA1), wasabi (TRPA1), and capsaicin (TRPV1).20, 21, 22, 23, 24, 25
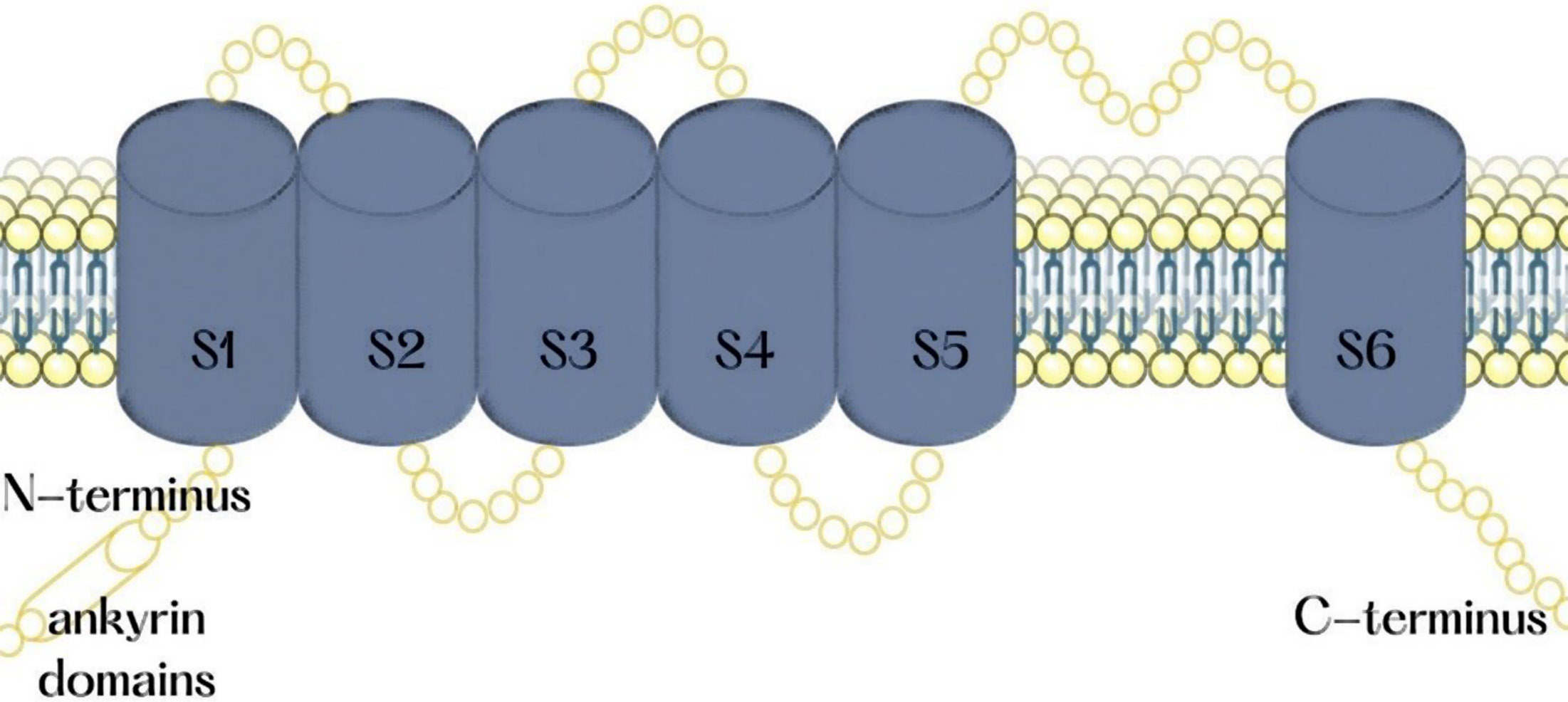
The TRPV1 is a non-selective cation channel that is permeable to calcium and is gated by noxious heat, vanilloids, extracellular protons, and endocannabinoids.18, 21 This channel is a tetrameric membrane protein that contains 4 indistinguishable subunits gathered around an aqueous pore in the central part of the channel.26 Each subunit of this multimeric protein consists of 6 transmembrane segments (S1, S2, S3, S4, S5, and S6) with an amphipathic region between the 5th and 6th segment that forms the channel conductive pore (Figure 2). Glutamic acid docks into the ampiphatic region in a pH-sensitive manner and gates the whole ionic current through the channel.21 In the cytoplasmic N-terminus of the protein, there are 3 ankyrin domains. These domains present consensus sequences for protein kinases and conciliate the protein-protein model of cytosolic protein interactions. The C-terminus domain includes phosphoinositide calmodulin binding (CAM) domains and phosphorylation sites.15 In addition, this receptor exhibits the TRP-like motif, which plays the role of an associative domain for the whole receptor to interact with other membrane-bound proteins.27
With regard to the involvement of TRPV1 in cancer, the receptor is now the most important target for the treatment of chronic pain in bone cancer.28 For this reason, the search for TRPV1 specific antagonists is becoming a promising direction in drug discovery and development.29 As a regulator of inflammation and calcium signaling pathways, the TPRV1 channel can affect the development and progression of cancer.30 The receptor is also upregulated in various neoplasms, including breast and urothelial cancers,31 and this relationship may find an application in the therapies targeting tumors.32 The anticancer potential of TRPV1 activation arises from an increased permeability of the cell membrane for chemotherapy agents.33, 34 The effects of receptor activation depend on the preliminary sensitization of TRPV1. The activation of TRPV1 in neurons leads to the release of pro-inflammatory substance P and calcitonin-related peptide (CGRP).35, 36 However, the summarized systemic effect of TRPV1 knockout turned out to be anti-inflammatory.37 Most of the studies aiming to evaluate the proliferation of cancer cells with the use of selective TRPV1 antagonists have shown that the inhibition of the channel is not related to a higher probability of cancer.38 In addition, a study by Hwang et al. showed that TRPV1 is not involved in carcinogenesis induced by activation of the Akt pathway.39
The other example are vanilloids, which induce TRPV1-unrelated apoptosis in oral cancer.40 Activation of calcium signaling has been widely evaluated for its potential application in the activation of the intrinsic apoptotic pathway in cancer.41 The assumption is that activation of calcium inflow to the cytoplasm and the mitochondria would result in programmed cell death. However, in the case of TRPV1, the effect varies between analyzed cell lines and, curiously, is related to the level of TRPV1 expression.31 Namely, breast cancer cell lines (MDA-MB231, MCF7) that express the TRPV1 channel are not sensitive to the administration of its selective agonist – vanilloids. However, after the transfection with the cDNA of the channel, the sensitivity increases significantly.
The biological effects of TRPV1 activation may also arise from its interactions with the other transmembrane proteins, especially those from TRP family.42 In the case of a therapeutic approach, selective inhibitors of TRPV1 have proven their efficacy in vitro. The application of a TRPV1 activator simultaneously with standard chemotherapy agents (cisplatin, 5-Fluoruracil, Pirarubicin), leads to a synergistic effect.43, 44, 45 The standalone effect of TRPV1 activation in thyroid adenocarcinoma cells has been proven effective as well.46
CK2α
Although casein kinase II (CK2) is not an oncogene, its activity is significant in various types of cancers, especially hematological malignancies.47, 48, 49 The antiapoptotic properties of this kinase are associated with an ability to sustain cancer growth. The CK2α subunit is the antiapoptotic protein by which vanillin might induce apoptosis in cancer cells through the inhibition of NF-κB phosphorylation and activation.50 An interaction between the receptor and 4-hydroxy-3-methoxybenzaldehyde inhibits the kinase function of CK2. A guaiacol functional group interacts with the positively charged region of the CK2α ATP blinding pocket. The potency of the inhibitory activity of vanillin is approximately equivalent to the effects induced by other substances, like feruloyl methane or ferulic aldehyde. The use of vanillin and its derivatives in the design of specific CK2 inhibitors may prove to be very successful.51 Protein kinase 2 is associated with the increased growth and proliferation of cancer cells.52 This kinase acts as a potent inhibitor of apoptosis, allowing the cells to proliferate. Moreover, increased expression is related to the level of dysplasia among cancer cells.53 The CK2 mechanism of action involves activation of the NF-κB pathway by the phosphorylation (and further degradation) of its inhibitor IκB.54 This kinase is also involved in the induction of drug resistance.55 In addition, CK2 inhibitors cause the suppression of angiogenesis56 and the cancer-specific, PTEN-related energetic shift.57 Various drugs have been tested for allosteric and ATP-competitive inhibition of CK2 in cancer therapy,58 with the latter including the small molecule inhibitors TBB, DMAT, IQA, CX-4945, and CX-5011.59 The ATP analogues contain the 2-aminothiazole-derived compounds.59 Moreover, some CK2 inhibitors have proven efficacious in preclinical studies, like CX4945 for high-risk pediatric leukemias.47 Aside from the novel chemotherapy agents, curcumin and its degradation products (ferulic acid, vanillin, feruloylmethane and coniferyl aldehyde) turned out to be potent CK2 inhibitors.51
CAMK4
Human calcium/calmodulin-dependent protein kinase IV (CAMK4), a member of the Ser/Thr kinase family, is associated with different types of cancer. Vanillin is considered a potential anticancer agent, and therefore its compatibility with the receptor binding pocket of this kinase has been investigated. It has been found that this molecule binds strongly to the active site cavity of CAMK4.60 As further research has shown, the anti-cancer potential of vanillin is related to its interaction with the described receptor, successfully inhibiting the proliferation of HepG2 (human hepatocyte carcinoma) and SH-SY5Y neuroblastoma cells.61 Moreover, vanillin treatment does not only affect tumor proliferation directly, but it also reduces ROS production and mitochondrial membrane depolarization, which eventually leads to the apoptosis in human hepatocyte carcinoma and neuroblastoma cells.62 Therefore, targeting CAMK4 as a novel therapeutic prospect might result in the common usage of vanillin (a natural chemical molecule) and its derivatives, which could be crucial when it comes to minimizing possible side effects.62 CAMK4 is also involved in the calmodulin-dependent protein kinase kinase 2 (CAMKK2) signal transduction pathway. Due to its activation of various transcription factors, neuronal communication and immune response, CAMK4 was considered a molecular target for anticancer therapy.63 Nuclear localization of CAMK4 is associated with the malignant potential of ovarian cancer.64 It has also been detected in lung and hepatocellular carcinomas.64 Indeed, hepatic cancer is essentially regulated by the CAMKK2/CAMK4 pathway.61 The CAMK4 has been examined as a potential target in the case of various malignancies, including hepatocellular carcinoma (HCC), breast cancer, neuroblastoma, prostate cancer, and acute myelogenous leukemia (AML).65 Vanillin has proven to be efficacious against cancer through binding to CAMK4 in HepG2 and SH-SY5Y cancer cells.62 Also, ellagic acid and quercetin were shown to inhibit the activity of this kinase.63 In addition, the natural inhibitor of CAMK4 – miR-129-5p – was found to inhibit MAPK and therefore the proliferation, migration and invasion of hepatic cells.66
MARK4
Microtubule affinity-regulating kinase 4 (MARK4) is a Ser/Thr kinase that belongs to the AMPK-like family.67, 68 This kinase regulates the stability of microtubules, and the cell cycle, signaling, differentiation and polarization69, 70; and its highest expression is observed in kidney, brain and testes.71, 72 Any fluctuations in MARK4 expression can disrupt important cellular pathways, such as mTOR and NF-κB, which may result in countless health disorders.73, 74 For instance, MARK4 has been reported to promote the proliferation of breast cancer cells through the hippo signaling pathway.74 Vanillin is considered to be a potential inhibitor of MARK4 and future anticancer research might target MARK4 overexpression.75, 76, 77 Therefore, exploring the interaction between MARK4 and vanillin may provide an effective tool to fight cancer.78 When it comes to the chemical structure and the process of binding, vanillin connects to this protein kinase only by a single hydrogen bond between A135 and the hydroxyl group of vanillin. However, alongside that connection, there is also a π–π bond with Y134.78 The MARK4 is overexpressed in different types of cancers, and this protein is responsible for the control of cell division in its early stages. This kinase regulates the microtubular system during cell division, and thus could be assigned as a target for novel drugs and naturally derived substances. Several studies have investigated the ability of natural compounds, like vanillin, rutin or rosmarinic acid, to act as inhibitors of MAPK4.78, 79 Also, several novel small molecule drugs have been shown to be potent MAPK4 inhibitors, exhibiting the potential of microtubule impairment for anticancer activity in MCF-7 and HepG2 cells.80 Dietary polyphenolics, ferulic acid, hesperidin, and gallic acid have also been shown to inhibit MARK4.78
Studies examining the use of vanillin to target cancers
The 4-hydroxy-3-methoxybenzaldehyde is considered to be generally non-cytotoxic. However, there is strong evidence confirming that this compound increases the cytotoxicity of cisplatin81 and mitomycin C,82 which are DNA-damaging agents. This fact is evidently related to the capability of vanillin to impair DNA double-strand break repair through the inhibition of DNA-PKcs.81 Even though this organic compound is incapable of directly suppressing the progression of a tumor, it might enhance the efficiency of chemotherapy, as suggested by Marton et al.,83 who showed that vanillin inhibits angiogenesis in a chorioallantois membrane assay of a chick.84 Moreover, vanillin suppresses the activation of NF-ĸB, which is induced by various inflammatory stimuli such as tumor necrosis factor α (TNF-α),50 trinitrobenzene sulfonic acid85 and 12-otetradecanoylphorbol-13-acetate.86 To conclude, even though the intrinsic cytotoxicity of vanillin is low, this organic compound can be used to sensitize cancer cells towards standard chemothrapeutic drugs, which results in the activation of NF-kB,83 and therefore may increase the effectiveness of these treatments.
In vitro studies concerning
vanillin receptors
The administration of 4-hydroxy-3-methoxybenzaldehyde in non-cytotoxic concentrations has shown anticancer potential in in vitro studies on mouse mammary adenocarcinoma 4T1 cells.87 Both invasion and migration were successfully inhibited by the described organic compound. Furthermore, experiments on human HepG2 cells have confirmed the usefulness of 4-hydroxy-3-methoxybenzaldehyde. Vanillin contributed to suppression of the enzymatic activity of matrix metalloproteinase 9 (MMP-9), induced by 12-O-tetradecanoylphorbol-13-acetate and decreased its mRNA level.86 The anticancer potential of vanillin was also shown in research on lung A549 carcinoma cells. The migration of these cells induced by hepatocyte growth factor (HGF) was successfully inhibited by vanillin.87 As demonstrated by these in vitro results, the inhibitory effects of vanillin on the activity of cancer-related proteins make it a very promising potential anticancer agent.
Vanillin is also considered a bio-antimutagen, as it prevents mutagenesis by reducing mutation progress after DNA damage.88 Studies by Rodrigues de Andrade et al. have shown that the application of 4-hydroxy-3-methoxybenzaldehyde decreases mitomycin C-induced and spontaneous ring X-loss.89 Even though mitomycin C-induced mutations were not effectively inhibited by vanillin, the proportion of recombination in somatic cells of Drosophila melanogaster that were treated with the alkylating agent increased significantly.90 When applied in combination with ethyl methanesulfonate (EMS), N-methyl-N-nitrosourea (MNU) or bleomycin, 4-hydroxy-3-methoxybenzaldehyde prevented the cellular genotoxicity induced by these chemical substances.91, 92 Furthermore, measurements of DNA repair have shown the impact of vanillin on the repair of lethal damage induced by N-ethyl-N-nitrosourea (ENU) and EMS.93 Finally, studies on the anti-mutagenic effects of 4-hydroxy-3-methoxybenzaldehyde have shown its inhibitory potential and stimulating effects on detoxification enzymes.92, 93
In contrast to the research cited above, the following studies were conducted on mammalian ovary fibroblast CHO K-1 cells. Apart from overall antimutagenic effects of 4-hydroxy-3-methoxybenzaldehyde, the influence of the current phase of the cell cycle on these effects was examined. The results showed that vanillin caused significant growth in the incidence of sister chromatid exchange (SCE) induced in cells treated with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) and methyl methanesulphonate (MMS), EMS and ENU. However, the effects obtained in cells treated with MMC were dependent on the cell cycle and occurred in the S phase.94 Also, vanillin treatment applied in the G2 phase in these cells decreased the frequency of breakage types of chromosome aberrations caused using X-ray radiation and ultraviolet (UV) light, whereas in phase G1, it suppressed both breakage and exchange types of aberrations induced with X-ray radiation.95 Even though the experiment was conducted successfully for several mutagens, in cells treated with N-ethyl-N′-nitro-N-nitrosoguanidine (ENNG) and mitomycin (MMC), the usage of vanillin did not produce significant changes. Cricetulus griseus lung fibroblast V79 cells have also been used to examine the suppressive properties of 4-hydroxy-3-methoxybenzaldehyde. Vanillin did not only minimize the incidence of 6-thioguanine-resistant mutations generated using ENU, X-ray radiation and UV light,96 but it also suppressed chromosomal aberrations evoked by hydrogen peroxide.97, 98
Human colon cancer HCT116 cells have also been used to study the antimutagenic capability of vanillin. The 4-hydroxy-3-methoxybenzaldehyde applied at antimutagenic concentrations caused DNA damage in mismatch-proficient (HCT116 + CHR3), as well as mismatch-deficient (HCT116) cells. In the end, the HCT116 cells treated with vanillin exhibited a change in the expression of 64 genes, mainly related to the DNA damage, oxidative damage, cell growth, and apoptosis.95 In addition, studies examining the effects of vanillin on DNA damage caused using UV light in human keratinocyte stem cells suggested that the ATM/p53 pathway relates to vanillin-induced protection of the cell.84
In vivo studies concerning
vanillin receptors
The first in vivo studies testing the anticancerogenic potential of vanillin were conducted in rats. These animal studies involved supplementing the diet with vanillin for 7 days and administering a hepatocarcinogen on the 6th day. Partial hepatectomy, as well as the application of phenobarbital and D-galactosamine, demonstrated antioxidant and inhibitory effects of vanillin on hepatocarcinogenesis initiation.99 Another study concerning medium-term multiorgan rat carcinogenesis used male F344 rats that were given vanillin in their diet, either from 1 day before and through the exposure to carcinogen, or afterwards. This study showed that 4-hydroxy-3-methoxybenzaldehyde suppressed the carcinogenesis of small intestine cancer and lung cancer, even though only for a week, whereas its effect on colon cancer cells in the initiation phase was strikingly different, and cancer cells progression was observed.100 Other studies have examined the effects of vanillin in rats with aberrant crypt foci (ACF) induced with azoxymethane.101 In these studies, the animals were given vanillin at either a low or a high dose, and several parameters, such as ACF density and distribution, as well as gene expression, were monitored. Although orally delivered 4-hydroxy-3-methoxybenzaldehyde did not yield any significant results, vanillin administered through intraperitoneal injection (at the higher concentration) was cancerogenic. However, the expression levels of many parameters were affected by the substance. For instance, the levels of the protooncogenes XRCC2, PMS2, p21, and cyclin B were increased.101 Both the cancerogenic and anticancerogenic effects of vanillin suggest that further studies are required in the field of vanillin-related changes in DNA.
Clinical trials involving vanillin
To date, several trials examining the use of vanillin in clinical practice have been conducted. Most of these studies have applied this natural compound for the treatment of hypoxic events102 or apneas103 in premature infants. In addition, the calming effects of vanilla odor have been tested for the potential induction of analgesia in preterm newborns.104 The repellent properties of vanillin have also been tested against flies, and thus its potential application for interrupting the transmission of trachoma has been examined.105 However, no trials have aimed to use vanillin as an anticancer therapy. Although vanillin has never been clinically applied in cancer patients, an extract from vanilla beans has been examined as a supportive/adjuvant therapy for cancer. Vanilla in nutraceuticals (especially flaxseed) has been tested for the prevention of doxorubicin- and trastuzumab-mediated cardiotoxicity.106 In the future, a registered clinical trial will evaluate its effects on the chemotherapy-induced nausea and vomiting (trial No. NCT04478630). The only study that concerned the standalone anticancer activity of vanilla was conducted on PSA-recurrent prostate cancer.107 This phase II clinical study revealed a decline in PSA slope after the administration of isoflavones (including vanilla).108 Although several studies aimed to evaluate the effects of whole food interventions (including vanilla) on mucositis in patients treated for thoracic109 and head and neck cancers,110 the studies were terminated early.
Conclusions
Substances of natural origin are effectively used in therapies, as these compounds tend to exhibit significantly less side effects. Even though our knowledge is still very limited, the anticancer potential of a widely accessible organic compound, vanillin, and the multitude of studies examining the role of vanilloid receptors in carcinogenesis, make the prospect of using vanillin and its derivatives in clinical trials very promising. The studies presented in this review reveal the antitumor activity of vanillin and its therapeutic potential in cancer treatment and prevention. The data reviewed above summarize the results of the most important research related to the role of 4-hydroxy-3-methoxybenzaldehyde and its derivatives as effective inhibitors of the pathophysiology of cancer. As it turns out, the described inconspicuous organic compound may be one of the substances that indirectly contributes to the inhibition of the tumor growth and it may become an effective treatment to combat the fatal consequences of carcinogenesis.