Abstract
Currently, there is no doubt that hepatitis C virus (HCV) infection is a systemic disease affecting not only the liver but also a range of other organs – extrahepatic manifestations (EHMs). Extrahepatic manifestations usually occur concurrently with liver disease, primarily have an immunological basis, and/or are a consequence of the direct impact of HCV on virtually all organs. The scope of the problem is significant; it has been shown that 30–40% of HCV-infected individuals are affected, which aligns with our own observations. Viral elimination (either spontaneous HCV clearance or as a result of pharmacotherapy) is crucial for the patient’s prognosis, both in terms of liver disease and EHM. Achieving a sustained virological response (SVR) only in many cases of EHMs is associated with remission of clinical symptoms of EHMs.
Key words: HCV, liver, mixed cryoglobulinemia
Introduction
The term “viral hepatitis C” is a significant oversimplification of the problem, as it does not encompass a range of highly diverse liver diseases associated with HCV, nor the numerous extrahepatic manifestations of this infection. Currently, there is no doubt that HCV infection is a systemic disease affecting not only the liver but also a variety of other organs – extrahepatic manifestations (EHMs). Extrahepatic manifestations typically occur alongside liver disease, are largely immunologically mediated, and/or result from the direct effects of HCV on multiple organs. Most experts believe that at least 1 form of EHM appears in 30–40% of those infected with HCV. Viral elimination (either spontaneous clearance of HCV or as a result of pharmacotherapy) is crucial for the patient’s prognosis, both in terms of liver disease – regardless of its stage – and extrahepatic manifestations. In many cases, achieving a sustained virological response (SVR) is associated with remission of clinical symptoms of EHMs. However, causal treatment initiated at a late stage of HCV-related disease often does not bring significant clinical benefits; hence, early diagnosis and therapy of HCV infection are of vital importance.1
Epidemiology
Infections caused by primary hepatotropic viruses – HBV, HCV, HAV, HEV, and HDV – are among the most serious infectious disease challenges in the modern world. According to the WHO, an estimated 50 million people are currently infected with HCV, compared with approx. 71 million a decade ago. In 2022, 242,000 deaths were attributed to chronic HCV infection, primarily from liver cirrhosis or hepatocellular carcinoma. Spontaneous elimination of HCV occurs in about 15–45% of infected individuals, usually within a few months, while the remaining 55–85% develop chronic infection. For untreated patients with chronic HCV, the risk of cirrhosis ranges from 15% to 30% within 20 years. The highest prevalence of HCV infection is observed in the Mediterranean region, China, India, Russia, as well as in Alaska and the USA. On the other hand, many countries – primarily wealthy, but not only European – are on a fast track to eliminate HCV by 2030, such as Canada, Australia, and Japan. The global decline in infections is due to advances in knowledge, improved sanitary and epidemiological standards, the use of disposable medical equipment, easier access to diagnostics – especially molecular techniques – and better availability of safe, well-tolerated antiviral therapies based on direct-acting antivirals (DAAs). In Poland, approx. 700,000 individuals have anti-HCV antibodies, but active viral replication (HCV RNA positive) is detected in only about 150,000. In 2024, according to NIZ-PZH data, just 3,526 cases of chronic HCV-related liver disease and 44 cases of acute hepatitis were diagnosed – figures that are highly concerning given the estimated number of individuals with active replication. Notably, the registry does not capture cases presenting with extrahepatic manifestations of HCV infection. At the current rate of diagnosis, elimination of HCV in Poland may not be achieved until around 2060, a timeline similar to that projected for the United States.1, 2, 3, 4, 5
Extrahepatic manifestations of HCV infection
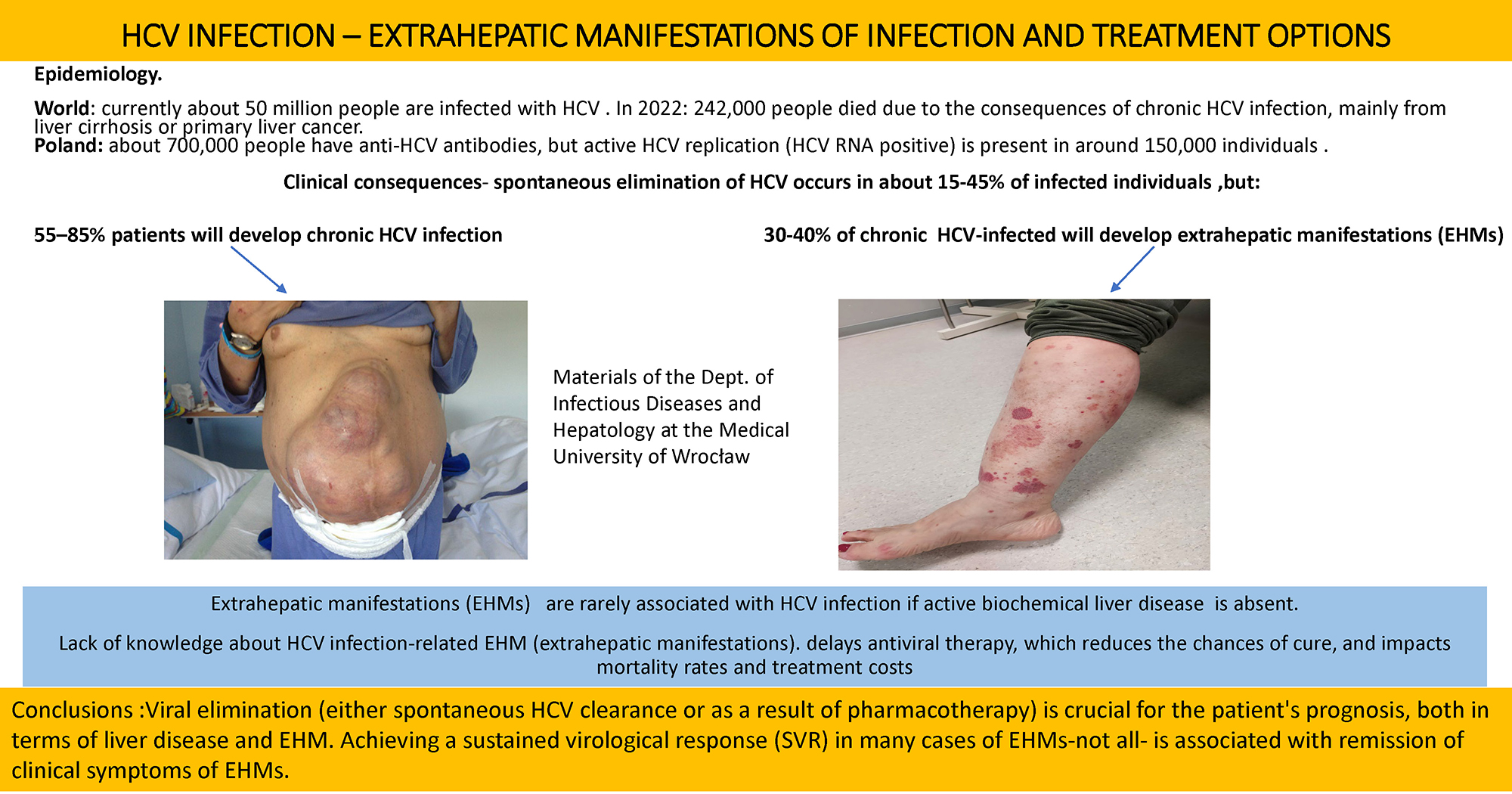
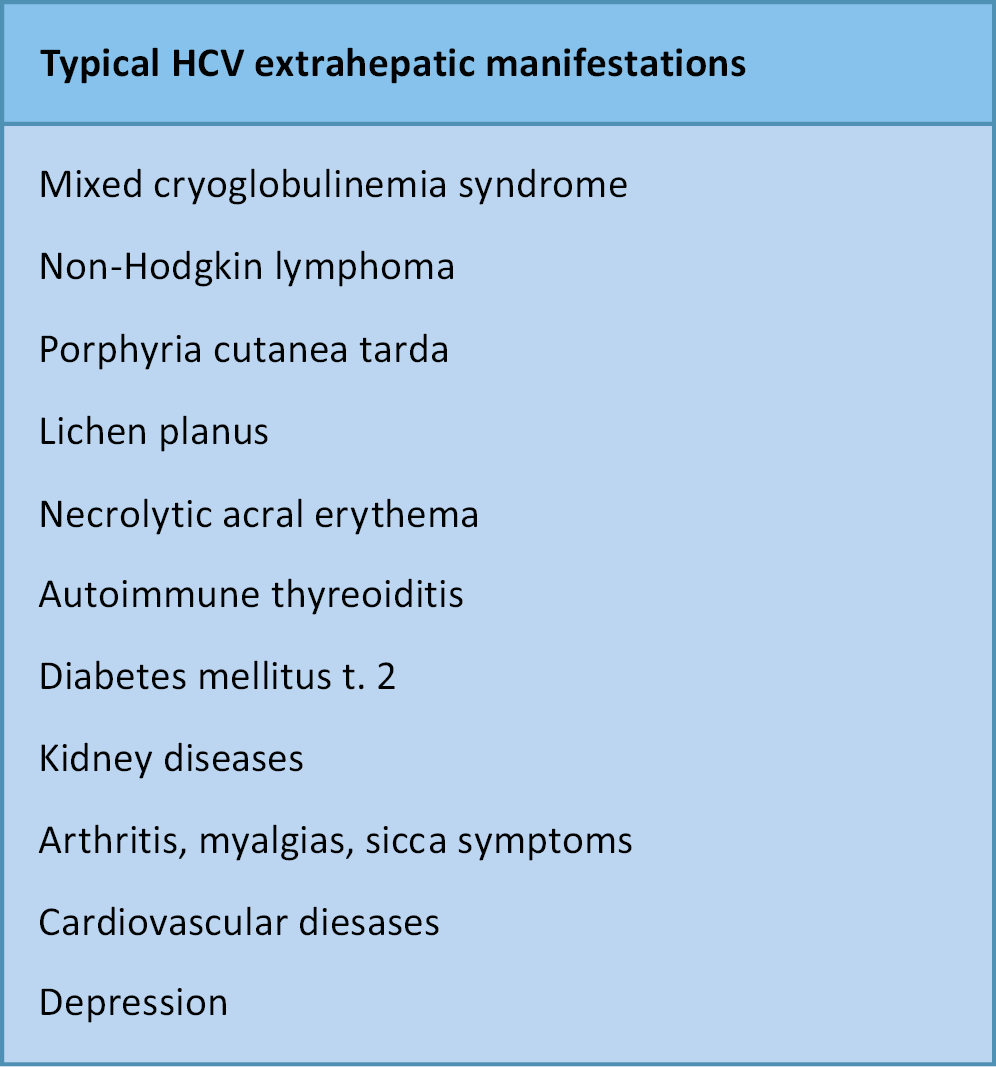
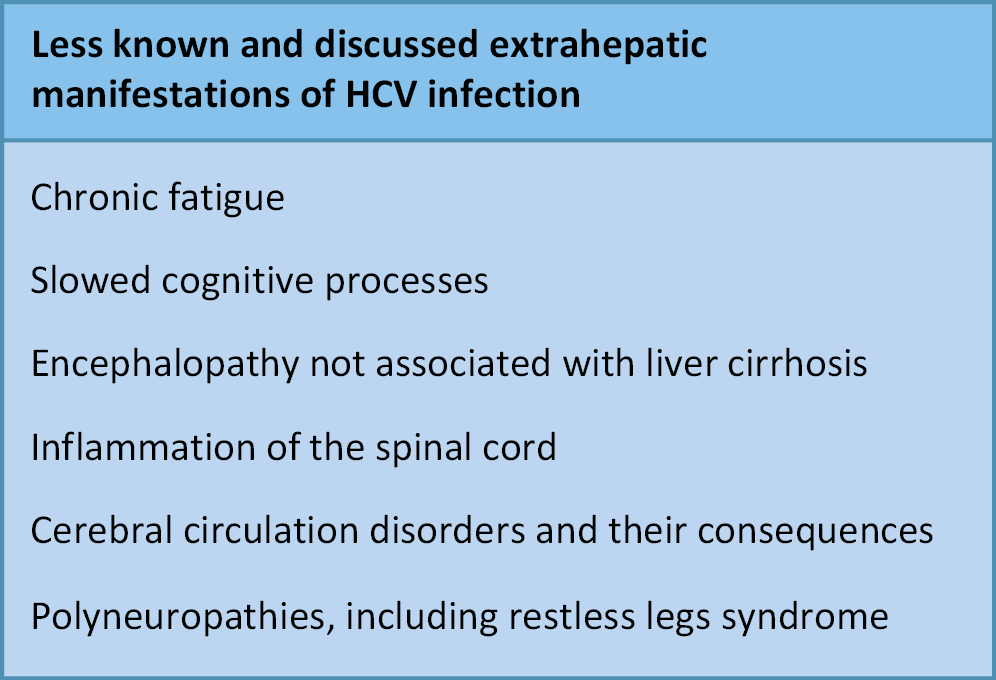
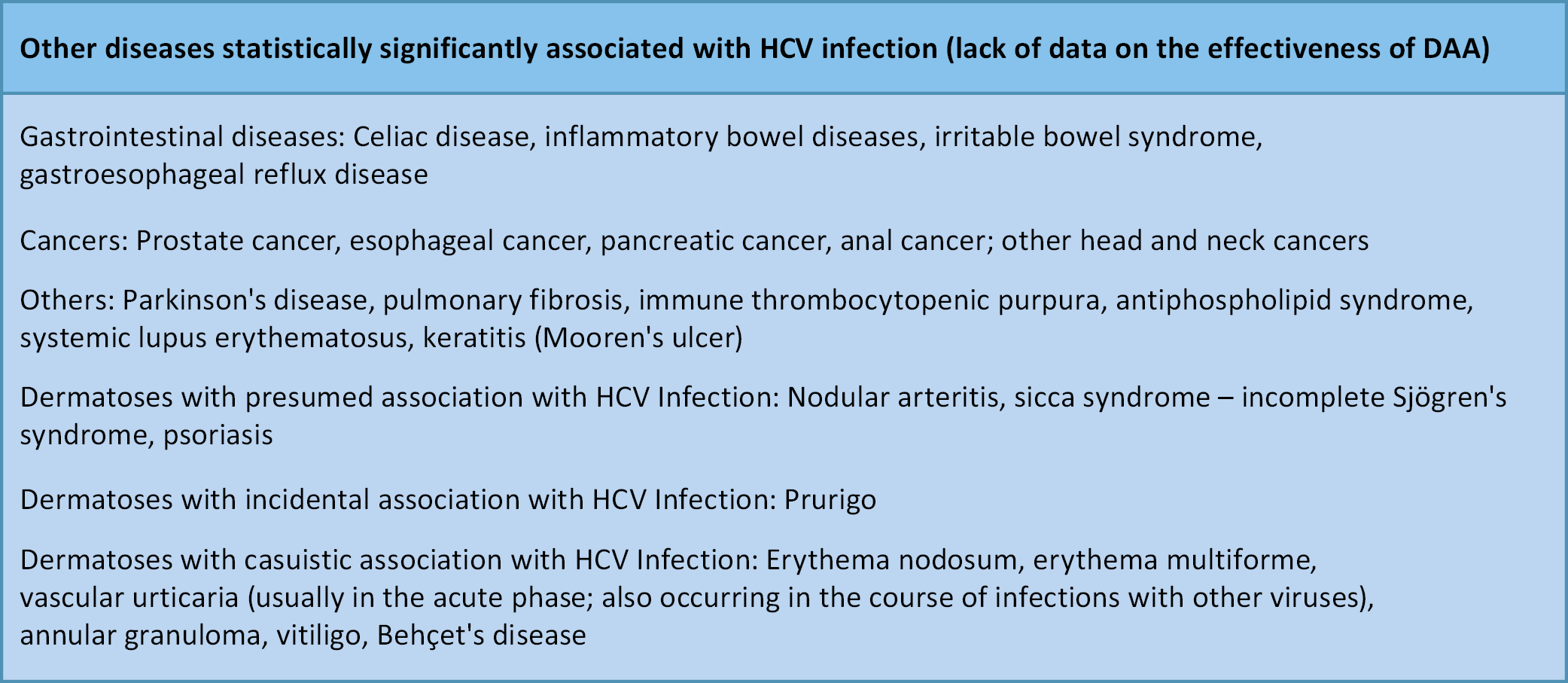
The hepatic manifestations of HCV infection are well characterized. In contrast, EHMs are often not linked to HCV in the absence of active biochemical liver disease. This frequently delays initiation of antiviral therapy, thereby reducing the likelihood of cure and adversely affecting mortality and treatment costs (Figure 1, Figure 2).6, 7, 8, 9 Moreover, numerous diseases involving different organs show a statistically significant association with HCV infection, although the nature of this relationship remains unclear. Does HCV infection increase the risk of these conditions? What immunological mechanisms are involved? (Figure 3).10, 11, 12, 13, 14, 15, 16, 17
HCV extrahepatic manifestations in details
Mixed cryoglobulinemia
Mixed cryoglobulinemia (MC), most commonly type II, is the most frequent EHM of HCV infection. It is defined by the presence of cryoglobulins–immune complexes that precipitate at temperatures below 37°C – and is associated with vasculitis and multiorgan involvement. Mixed cryoglobulinemia occurs approx. 11 times more often in HCV-infected individuals than in uninfected ones, affecting about 4.9% of patients with HCV. Notably, around 90% of patients with cryoglobulins have detectable anti-HCV antibodies and HCV RNA, confirming active infection. Mixed cryoglobulinemia is both an autoimmune and lymphoproliferative disorder, resulting from persistent activation of B lymphocytes by HCV to produce antibodies. Clinically, MC is usually mild, but in 5–10% of cases it progresses to non-Hodgkin lymphoma, with a risk 35 times higher than in the general population. Cryoglobulins precipitate and deposit in the walls of small- and medium-sized blood vessels, leading to vasculitis. The organs most commonly affected are the skin, joints, kidneys, central and peripheral nervous systems, gastrointestinal tract, and heart. Typical symptoms include weakness, purpura (skin hemorrhages), and joint pain, usually without true arthritis. The joints most frequently affected are the proximal interphalangeal and metacarpophalangeal joints of the hands, as well as the knees and hips. Renal involvement is manifested by proteinuria and hematuria, which may progress to membranoproliferative glomerulonephritis and renal failure. Neurological symptoms include paresthesias, tingling, and burning sensations in the feet and hands, reflecting peripheral polyneuropathy, while isolated mononeuropathies are uncommon. In severe cases, MC can progress rapidly, leading to renal failure, intestinal ischemia, and pulmonary hemorrhage.
Treatment involves DAAs. Achieving SVR leads to remission of symptoms in about 70% of treated patients. In patients with clinically severe MC, unfortunately, the pharmacological elimination of HCV does not affect the course of this condition. This group of patients requires immunosuppressive treatment.6, 8, 9
Non-Hodgkin lymphomas
The risk of developing B-cell non-Hodgkin’s lymphoma (B-NHL) is increased by 28% among individuals infected with HCV compared to uninfected persons. A meta-analysis based on 48 clinical studies demonstrated that HCV infection is present in approx. 15% of patients with B-NHL. Furthermore, 90% of patients with NHL and circulating cryoglobulins have detectable HCV viremia. The most common subtypes of NHL associated with HCV infection include: diffuse large B-cell lymphoma (DLBCL), lymphoplasmacytic lymphoma (Waldenström’s macroglobulinemia), marginal zone lymphoma (MZL) – particularly splenic marginal zone lymphoma (SMZL) and nodal marginal zone lymphoma (NMZL) – and MALT lymphoma. It has been shown that 2/3 of HCV-associated lymphomas tend to have a less aggressive clinical course. Approximately 10% of HCV-infected patients develop monoclonal gammopathy, characterized by the production of IgM kappa antibodies, which may indicate an increased risk of multiple myeloma. HCV infection can also be associated with polyclonal or oligoclonal hypergammaglobulinemia, most often involving IgG, which correlates with the severity of hepatitis. Coexisting chronic hepatitis further increases the risk of hepatotoxicity during lymphoma therapy, particularly with rituximab..
Treatment: DAAs should be considered as first-line treatment in cases of indolent lymphomas. For more aggressive lymphomas (intermediate and high-grade), initial treatment with immunochemotherapy followed by antiviral therapy is recommended. It has also been demonstrated that effective HCV treatment (achieving sustained virologic response, SVR) statistically reduces the risk of developing NHL in previously infected patients.6, 8, 9, 10, 11, 12, 13
Dermatological diseases
Compared with uninfected individuals, HCV infection increases the risk of porphyria cutanea tarda (PCT) more than 8.5-fold and of lichen planus (LP) approx. twofold, particularly in patients with HCV genotypes 1 and 4. Both PCT and LP occur in 1–2% of HCV-infected individuals, independent of the cutaneous manifestations seen in MC.
Treatment: Effective antiviral therapy reduces the severity of PCT symptoms. However, its impact on the incidence and clinical course of lichen planus has not been demonstrated. Necrolytic acral erythema, a rare skin condition associated with HCV and clinically resembling psoriasis, may recur even after successful antiviral treatment. The association between other dermatological diseases and chronic HCV infection remains unclear.6, 8, 9, 10, 11, 12, 17
Endocrine diseases
Type 2 diabetes and insulin resistance occur in up to 15% of HCV-infected individuals and are more than 1.5 times more common than in uninfected populations. HCV is an independent risk factor, conferring a 70% higher likelihood of developing insulin resistance and type 2 diabetes. Impaired glucose metabolism, in turn, accelerates the progression of liver cirrhosis, increases the risk of hepatocellular carcinoma, and contributes to cardiovascular complications. Antiviral therapy with DAAs reduces the incidence of insulin resistance and type 2 diabetes, while in patients with pre-existing diabetes, it improves glycemic control. Achieving sustained virological response (SVR) lowers the risk of vascular complications, including end-stage renal disease (by 84%), ischemic stroke (by 47%), and acute coronary syndromes (by 36%).
Thyroid disorders, most commonly autoimmune conditions such as Hashimoto’s thyroiditis and Graves’ disease, constitute the 2nd major group of endocrine diseases associated with HCV infection. They occur in 2–13% of HCV-infected individuals, most frequently presenting as hypothyroidism. An increased risk of papillary thyroid carcinoma has also been reported in this population.
Treatment: Antiviral therapy with DAAs is considered the standard approach for all forms of HCV-associated thyroid disease.6, 10, 12
Kidney diseases
Compared with uninfected individuals, those with HCV infection have an approx. 26% higher risk of developing and progressing kidney disease. The clinical spectrum is diverse – about 10% of HCV-infected individuals eventually develop renal manifestations, most commonly membranoproliferative glomerulonephritis (MPGN) and, less frequently, nephrotic syndrome. Membranoproliferative glomerulonephritis is typically associated with mixed cryoglobulinemia.
Treatment: The first-line therapy is antiviral treatment with DAAs. Effective antiviral therapy reduces proteinuria, although it usually does not result in complete resolution of kidney disease. In cases with rapidly progressive renal deterioration, immunosuppressive therapy remains the primary treatment approach.6, 9, 12
Arthritis, myalgias, sicca symptoms
The prevalence of musculoskeletal symptoms (myalgia and arthralgia) and sicca syndrome among individuals infected with HCV is more than twice as high as in uninfected individuals. Joint pain occurs in 6–20% of patients, while arthritis is observed in a significantly smaller percentage of HCV-infected individuals (about 4–5%). In patients with circulating cryoglobulins, joint pain is more frequently observed, affecting even 40–80% of those infected with HCV. Arthritis is usually polyarticular, symmetrical, and involves the joints of the hands, knees, ankles, and spine. Clinically, it often resembles rheumatoid arthritis, but without accompanying joint surface erosion and deformities. The detection of rheumatoid factor may lead to misdiagnosis of rheumatoid arthritis, as it is also present in 40% of HCV-infected individuals. Approximately 10–30% of individuals infected with HCV experience symptoms related to dryness of the mouth, eyes, and genital organs. Significantly fewer, about 5%, have Sjögren’s syndrome defined by the presence of specific antibodies (anti-SSA and anti-SSB) and histological changes.
Treatment involves the use of DAAs, regardless of immunosuppressive and biological therapy.6, 8, 10
Cardiovascular diseases
The risk of cardiovascular complications (ischemic heart disease, chronic heart failure, ischemic stroke, carotid artery atherosclerosis) in patients infected with HCV is approx. 20% higher than in the uninfected population, particularly in those with active HCV replication (HCV RNA positive). This also applies to patients with HIV/HCV co-infection, which presents a more complex issue. There are reports from Japan linking HCV infection with myocarditis and cardiomyopathy. The presence of HCV has also been demonstrated within atherosclerotic plaques. Undoubtedly, these relationships result from the influence of many not fully understood factors associated with chronic HCV infection, including endothelial dysfunction and low-grade systemic inflammation.
Treatment with DAAs significantly reduces the risk of developing cardiovascular diseases.8, 10, 11, 12
Neuropsychiatric disorders
The HCV infection has been shown to be associated with more than a twofold increased risk of a range of neuropsychiatric disorders, including mood disorders, depression, cognitive dysfunction, sleep disturbances, and chronic fatigue syndrome. There is also evidence of HCV’s involvement in demyelinating processes, functional and organic brain disorders, and even a connection with certain forms of encephalitis. Various forms of depression occur in 24.5% of individuals infected with HCV, while sleep disturbances and chronic fatigue affect as many as 60%. In our observations, these issues are seen in nearly all patients infected with HCV.
Treatment: DAAs should be considered as first-line treatment in milder cases and in the course of neurological diseases of vascular and demyelinating origin, particularly those associated with cryoglobulinemia. Achieving SVR is associated with the resolution of neuropsychiatric symptoms, mainly chronic fatigue symptoms, and allows for the discontinuation of antidepressant medications in some patients.10, 14, 15, 16
Treatment summary: Extrahepatic manifestations of HCV infection
Elimination of the virus through pharmacotherapy with DAAs is crucial for patient outcomes in both liver disease and EHMs. In most, though not all, cases, achieving a sustained virological response (SVR) is associated with remission of EHM symptoms. When treatment is initiated at an advanced stage of HCV-related disease, however, clinical benefits are often limited, underscoring the importance of early diagnosis and therapy. In a large cohort of more than 25,000 individuals with HCV in British Columbia, Canada, DAA treatment significantly reduced EHM-related mortality risk by 78–84%.18, 19, 20, 21, 22, 23
Conclusions
Chronic HCV infection is associated with a wide spectrum of clinical conditions beyond liver disease. Antiviral therapy with DAAs, irrespective of its effect on liver disease regression or progression, has a significant impact on the remission of selected EHMs. In addition, successful DAA therapy improves quality of life and positively affects overall wellbeing.18, 19, 20, 21, 22, 23
Use of AI and AI-assisted technologies
Not applicable.