Abstract
Keratin biomaterials, derived from natural sources, offer a promising, biocompatible solution for wound healing and tissue regeneration, though further clinical studies are needed to confirm their efficacy.
Key words: biomaterials, keratin, wound healing, wound dressing
Introduction
Impaired wound healing is a major medical problem, especially in diabetic patients. Among the numerous complications of diabetes, wound healing disorders are particularly noteworthy, severely impacting patients’ quality of life and placing a considerable burden on healthcare resources.1, 2 Researchers worldwide are seeking new strategies for wound care, especially in diabetic conditions. Considering this, several natural biomaterials, including keratin, silk, chitosan, alginate, and collagen, have emerged as promising candidates due to their biomedical properties.3, 4, 5, 6 Among these, keratin-based biomaterials have emerged as promising candidates due to their biocompatibility, biodegradability and ability to promote cell growth.3, 4, 7, 8
This editorial compiles insights from various preclinical and clinical studies on the use of keratin-based biomaterials for wound healing.
The biological effects and role of keratin
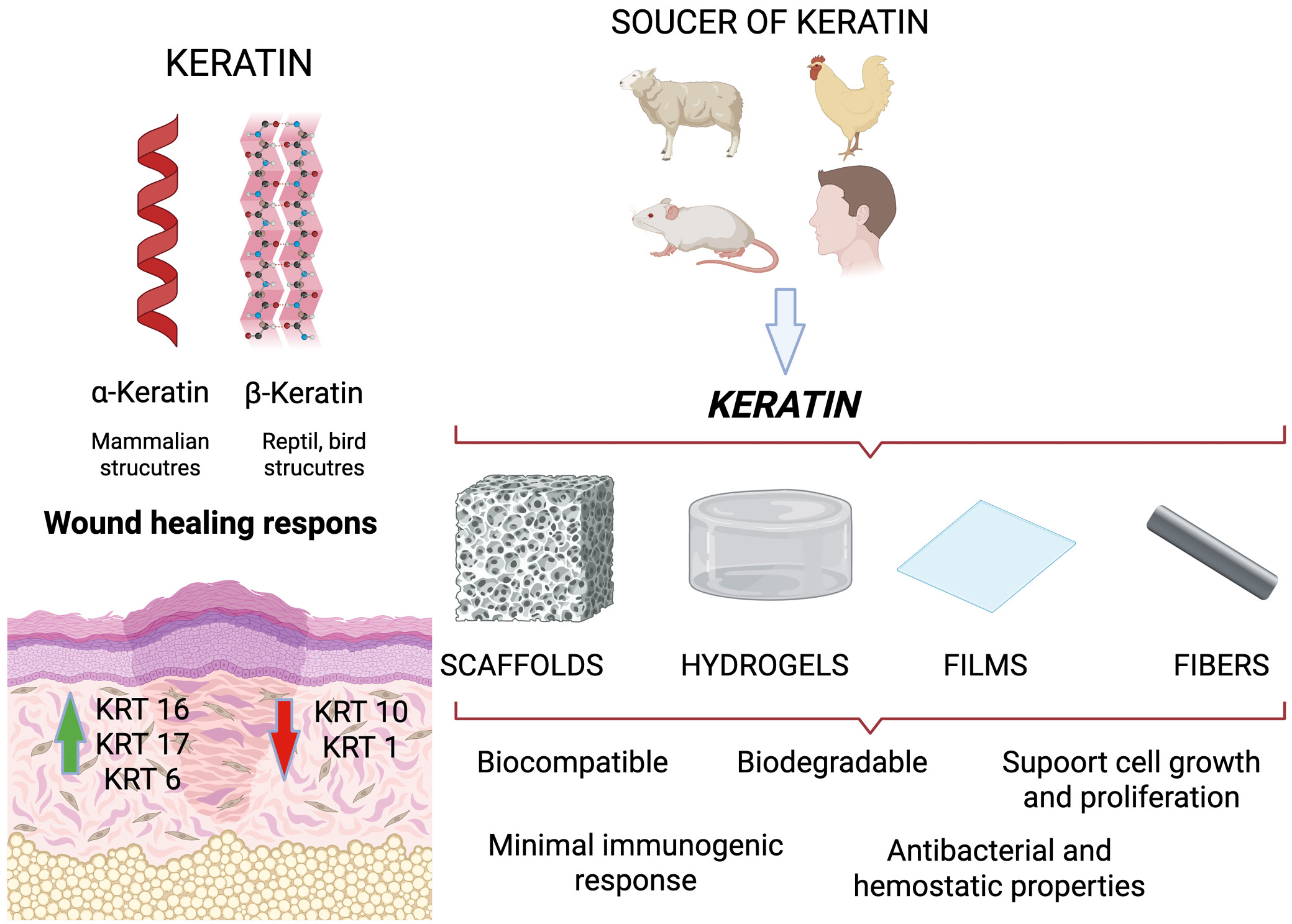
Keratins, a group of insoluble fiber-forming proteins, are found mainly in some vertebrate epithelial cells. It is formed through the copolymerization of 19 amino acids at the basic structural level.9 Keratin is rich in cysteine (17.5%), serine (11.7%), glutamic acid (11.1%), threonine (6.9%), glycine (6.5%), arginine (5.6%) and proline (5.6%), but with low levels of lysine, histidine and methionine, with tryptophan barely present.10 Keratin can be divided into 2 main types based on its molecular structure: α-keratin and β-keratin. α-keratin is mainly found in mammals and is a basic component of structures such as wool, hair, nails, hooves, and horns.11 It has a helical (spiral) structure made up of α-helixes and is known for its elasticity and strength. On the other hand, β-keratin is found in reptiles and birds, including in feathers, beaks, claws, and scales.12 Its β-sheet structure makes it stiffer and stronger than α-keratin. Additionally, keratins can be categorized as soft or hard based on their physical properties and sulfur content. Hard keratins are more rigid due to their higher sulfur levels.12 Furthermore, keratins are proteins that form a protective layer for the epidermal appendages, thus playing an essential role in protection. Keratin genes account for the majority of intermediate filament genes in the human genome, forming the 2 largest groups of sequence homology: type I keratins (also known as acidic keratins, comprising KRT9–KRT40), and type II keratins (also known as neutral-base keratins, comprising KRT1–KRT8).7, 13, 14, 15, 16
Activated keratinocytes produce both types of keratin, which are encoded by 54 evolutionarily conserved genes: 28 type I and 26 type II. These genes are expressed in tightly regulated pairs, depending on tissue type, cellular differentiation and physiological context.15, 16 The specific combinations of keratin monomers vary depending on the epidermal layer. For example, the KRT14-KRT5 pair is expressed mainly in the basal layer of the epidermis, while the KRT10-KRT1 pair is more abundant in the suprabasal layers of the epidermis.17 Following injury, stressed keratinocytes rapidly induce the de novo transcription of KRT16/17-KRT6. This is usually limited to the cells of the epidermis in smooth skin, the oral mucosa and several appendages.18, 19 KRT16/KRT17-KRT6 keratin pairs play a key role in wound healing. In the first few hours after injury, their expression is significantly increased. At the same time, a decrease in KRT10-KRT1 expression is observed. This change promotes keratinocyte proliferation over keratinocyte differentiation, thus facilitating rapid reepithelialization.19, 20 Studies using null mice have shown that a deficiency in KRT6a/KRT6b leads to fragile skin, imbalanced keratinocyte stability and defective wound healing. This is due to disrupted KRT6/KRT16 heteropolymer formation and reduced SRC kinase inhibition.21, 22, 23 In case of KRT-17, it has been demonstrated that KRT-17 null keratinocytes are reduced in size and demonstrate a decreased protein translation rate, which correlates with reduced mTOR/AKT signaling and directly influences keratinocyte proliferation.24
Keratin and keratin-based biomaterials gain much attention in the biomaterials world due to their interesting set of properties, including excellent biocompatibility, biodegradability, bioactivity, and minimal immunogenic response (Figure 1).7, 25, 26 In addition, these materials present a hydrophilic surface, which is not commonly found in synthetic polymers.27 Moreover, keratin-based biomaterials derived from wool and hair have been shown to facilitate cellular attachment, due to the presence of cell-binding motifs, including glutamic acid–aspartic acid–serine (EDS), arginine–glycine–aspartic acid (RGD), and leucine–aspartic acid–valine (LDV) residues.28, 29, 30, 31 On the other hand, the presence of RGD-binding residues acts as a binding motif for proteins such as fibronectin and fibrinogen. There are also studies describing the antibacterial and hemostatic properties of keratin biomaterials.32, 33, 34, 35, 36 The aforementioned properties enable scientists to create various types of wound-healing materials that support the healing process.
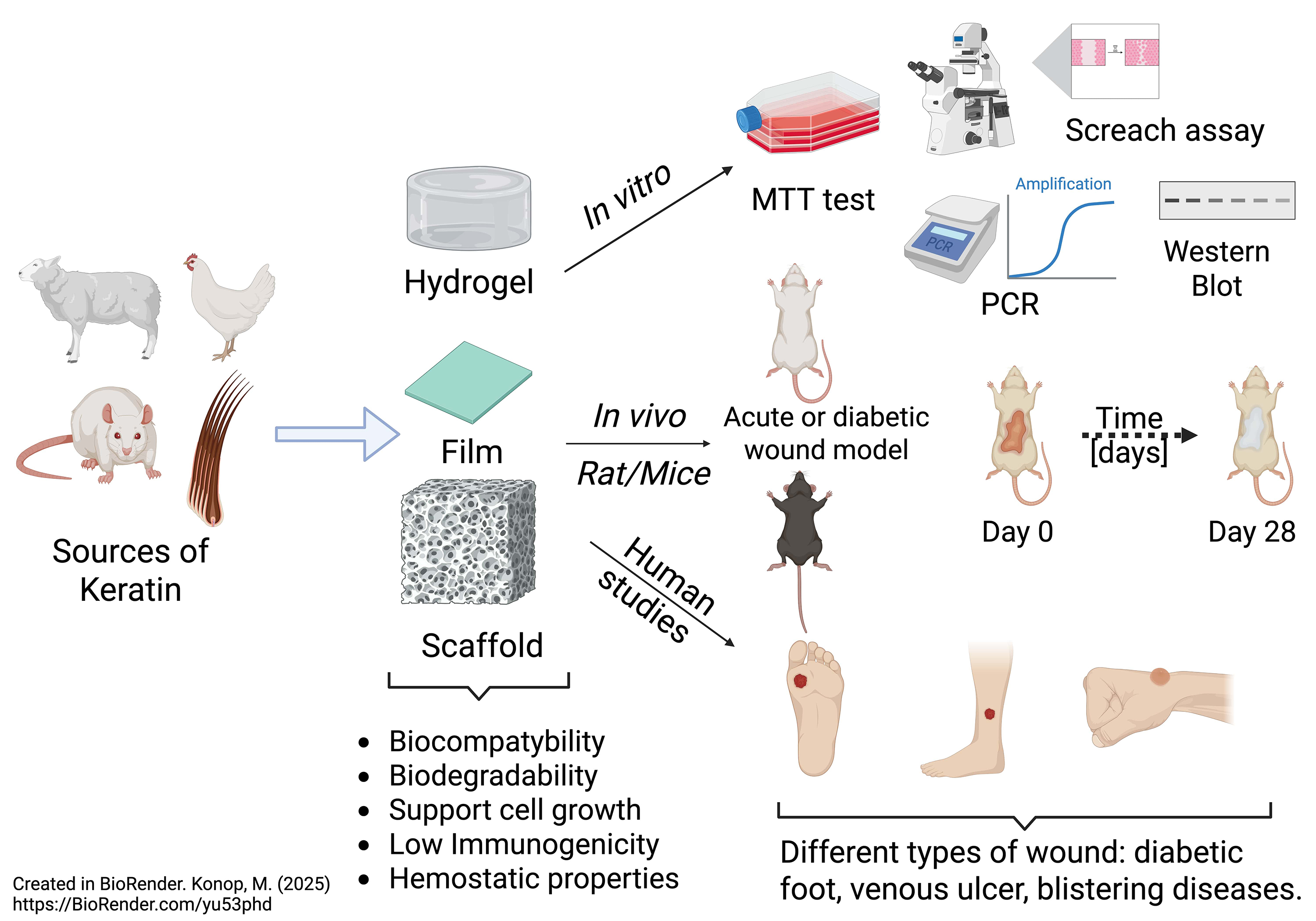
Preclinical study
Naturally derived biomaterials, such as keratin, have recently garnered significant attention in dermatology and regenerative medicine. Researchers commonly use soluble or insoluble forms of keratin biomaterials as wound dressings in preclinical studies involving various animal models of wound healing. They have also used keratin-based biomaterials to create hydrogels, films, nanofibers, and scaffolds as wound dressings in wound care.35, 37, 38, 39
Jelodari et al.40 examined keratin-based hydrogel dressing and its impact on delivering LL-37 antimicrobial peptide using full-thickness rat wounds. The L-KO25:KN75 hydrogel, a 25:75% ratio of keratose/kerateine, demonstrated significant human dermal fibroblast proliferation (~85% on day 7) and high adhesion (~90 cells per high-power field). It promoted enhanced migration with nearly complete wound closure within 24 h in a “scratch assay”. Microbiological examination showed effective antibacterial activity against Escherichia coli and Streprococcus aureus, reducing bacterial growth by over 70%. In vivo studies in Wistar rats indicated superior wound healing (~80% on day 14, >98% on day 21), outperforming other hydrogels. Histological analysis revealed increased angiogenesis, collagen deposition and skin regeneration. Increased mRNA expression of vascular endothelial growth factor (VEGF) and interleukin 6 (IL-6) further supported wound healing. The results summarize that LL-37-loaded keratin hydrogels can promote wound healing by enhancing tissue regeneration and reducing bacterial infections.
Han et al.38 also investigated a hydrogel composed of strontium ranelate-loaded human hair keratin and hyaluronic acid, utilized as a wound dressing in a full-thickness skin defect model in Sprague Dawley rats. In vitro examination revealed that incorporating strontium ranelate (SrR) into a hydrogel formulation significantly mitigated oxidative stress in murine fibroblasts (L929) and augmented the anti-inflammatory response in lipopolysaccharide-stimulated RAW264.7 macrophages. In vivo analyses further demonstrated that, relative to controls, the K/HA/0.5 mM SrR-treated group exhibited marked reductions in reactive oxygen species (ROS), IL-6 and tumor necrosis factor alpha (TNF-α), to 31.6%, 39.7%, and 61.1% of control levels, respectively. Moreover, in a full-thickness dermal wound defect model, the K/HA/0.5 mM SrR hydrogel significantly promoted wound closure by reducing oxidative injury, attenuating the inflammatory response and enhancing microangiogenesis.
Wang et al.41 explored the wound-healing potential and biocompatibility of a hydrogel derived from chicken feather keratin. The hydrogel, created through the cross-linking of keratin with H2O2, demonstrated a high porosity range of 77–82% and exhibited solid-like rheological properties, particularly at a concentration of 20%. The full-thickness wound model in Sprague Dawley rats demonstrated accelerated wound closure (~90% by day 10 vs ~60% in controls). Complete reepithelialization was achieved within 21 days after injury. Histological examination showed enhanced collagen deposition and neovascularization in treated wounds. Subcutaneous implantation of hydrogel revealed no systemic toxicity, organ damage or inflammatory response. The hydrogel degraded fully within 4 weeks. In conclusion, these findings suggest that feather keratin hydrogel could be used as a bio-dressing to speed up wound healing.
Zhang et al.42 developed and examined a novel bilayer wound dressing composed of poly(L-lactate-caprolactone) (PLCL) nanofibrous and keratin hydrogels, loaded with FGF-2 using a low-pressure filtration-assisted method. The keratin hydrogel mimicked the dermis with high porosity and a swelling ratio (874.09%), while the PLCL nanofibers simulated the epidermis due to their toughness and flexibility. In vitro examination using L929 cell line confirmed the biocompatibility and non-toxicity of the prepared dressing. In vivo studies on a full-thickness wound model in Sprague Dawley rats demonstrated accelerated wound healing in FGF-2-loaded dressings, with significantly higher re-epithelialization, collagen deposition, hair follicle regeneration, and angiogenesis compared to the control site (p < 0.05). The study concluded that the PLCL/keratin-FGF-2 bilayer dressing is a promising candidate for advanced wound care and tissue engineering applications.
In another study, Sun et al.43 investigated a keratin-based hydrogel incorporating basic fibroblast growth factor (bFGF) for diabetic wound healing in a full-thickness skin defect model in type II diabetic rats. In vitro studies using L929 fibroblasts showed that the K31/bFGF hydrogel enhanced cell viability and migration compared to controls. In vivo application of the hydrogel significantly accelerated wound closure, with the K31/bFGF-treated group exhibiting improved healing rates by days 7 and 14 (p < 0.001), greater epidermal thickness, increased neovascularization, and more abundant skin appendages. Mechanistically, the hydrogel promoted epithelial–mesenchymal transition via upregulation of vimentin and downregulation of E-cadherin, and activated the PI3K/Akt signaling pathway. These findings suggest that the K31/bFGF hydrogel is a biocompatible and effective wound dressing for enhancing diabetic wound repair.
Chen et al.44 created a glucose-triggered, in situ keratin hydrogel to treat diabetic wounds by employing a glucose oxidase-catalyzed disulfide shuffling approach. In vitro evaluations showed improved mechanical characteristics, low cytotoxicity using uman umbilical vein endothelial cells (HUVECs) and L929 mouse fibroblast cell line. In vivo experiments on streptozotocin-induced diabetic Sprague Dawley rats showed that the keratin-deferoxamine (DFO) hydrogel significantly accelerated wound closure on days 9 and 15 (p < 0.01). Moreover, keratin-DFO hydrogel facilitated reepithelialization, increased epidermal thickness and increased collagen formation/deposition. Additionally, immunofluorescence and immunohistochemical analyses indicated elevated angiogenesis through increased CD31 and VEGF labelling. In conclusion, the results obtained confirm that the keratin-DFO hydrogel is biocompatible, promotes the formation of new vessels and accelerates diabetic wound healing.
Khaliq et al.45 examined multifunctional hydrogel membranes made from keratin-pullulan-polyvinyl alcohol (KR-PL-PVA) and infused with cefotaxime sodium (CTX) for diabetic wound healing. In vitro cytotoxicity assays performed using NIH3T3 fibroblast cells confirmed high biocompatibility, with cell viability exceeding 95%. In vivo experiments conducted on streptozotocin-induced diabetic rats revealed notably faster wound healing rates, with the CTX-KR-PL-PVA group achieving 89% wound closure by day 14 and over 99% by day 21 (p < 0.05). Histological evaluations showed improved reepithelialization, dense collagen formation, developed skin appendages, and reduced inflammation. These findings suggest that CTX-infused KR-PL-PVA membranes are effective, biocompatible wound dressings demonstrating strong antibacterial properties and significant potential for diabetic wound treatment.
Mirhaj et al.46 prepared and examined mupirocin-loaded core-shell nanofibers using Pluronic F127, pectin, and keratin as an advanced wound dressing material. In vitro assays demonstrated that the sustained release of mupirocin from the core-shell fibers significantly improved human keratinocyte viability and proliferation compared to blended fibers, while also enhancing angiogenic activity in the CAM assay. In vivo tests using a full-thickness wound model in Wistar rats showed that wounds treated with the core-shell dressing achieved a 97.66% closure rate by day 14, significantly outperforming other formulations (p < 0.05). Histopathological and immunohistochemical examination confirmed accelerated reepithelialization, increased skin appendage formation and greater collagen deposition and vascularization. In conclusion, the F127-Mup/Pec-Kr core-shell nanofibers exhibit excellent biocompatibility, antibacterial efficacy and enhanced regenerative properties, suggesting strong potential as an effective wound dressing for chronic and ischemic wounds.
Radu et al.47 prepared and tested bacterial cellulose-keratin scaffolds, with and without adipose tissue-derived stem cells, as potential dressings for burn wounds. In vitro cytocompatibility tests using adipose tissue-derived stem cells showed increased cell viability (115%) after 72 h, indicating enhanced cell proliferation and biocompatibility. In vivo experiments on burn wounds in rabbits showed favorable healing progress at days 7, 14 and 21 after transplantation. In particular, wounds treated with the keratin-bacteria-cellulose scaffold with stem cells showed reduced inflammation, earlier regeneration of blood vessels and connective tissue, and more advanced re-epithelialization by day 21. In conclusion, the keratin-bacteria-cellulose scaffold promotes cell growth, accelerates tissue regeneration and significantly improves wound healing. Moreover, it is a promising dressing for treating burns when combined with stem cells.
Konop et al. examined keratin scaffolds as a wound dressing in healthy and diabetic conditions.5, 19, 26, 35 They showed that fur keratin-derived powder (FKDP) supports cell proliferation and migration. Moreover, they modified the obtained FKDP with antibacterial (AgNP)35, anti-inflammatory (butyrate)19 and analgesic substances (casomorphin).48 In vivo experiments showed that wounds treated with different FKDP-wound dressings healed significantly faster than control wounds. Moreover, they found that the obtained variants of FKDP were naturally incorporated into regenerated tissue and exhibited tissue biocompatibility and biodegradability. Immunofluorescence examination revealed that in all wounds treated with the examined dressing, M2 macrophages were predominant.19, 48 This group’s results showed the possible use of insoluble fractions of keratin biomaterials as wound dressings in clinical settings.
Rybka et al.5 examined keratin scaffolds containing biphalin as a potential wound dressing in a full-thickness wound model in diabetic mice. In vitro examination using NIH/3T3 cells showed increased cell viability and upregulation of p-AKT/mTOR signaling. In vivo experiments on diabetic C57BL/6J mice demonstrated that wounds treated with the keratin-biphalin dressing healed faster on days 5 and 15 post-surgery (p < 0.05), showing improved reepithel-ialization, thicker epidermis and enhanced macrophage infiltration. In conclusion, keratin-biphaline fibers are biocompatible, promote cell growth and significantly accelerate wound healing, indicating their promising properties as a natural dressing for diabetic wounds. In another study Rybka et al.25 examined keratin scaffolds containing trimethylamine N-oxide (TMAO) as a wound dressing in diabetic rats. They showed that keratin-TMAO wound dressing is safe, non-toxic and biocompatible in vitro and in vivo. In vivo examination showed that treated wounds healed significantly faster on days 4, 7, 14, and 21 post-injury (p < 0.05). Immunofluorescence and histopathological examinations showed that in dressed wounds predominant M2 macrophages, which are responsible for faster wound healing. Furthermore, the study revealed in vitro an enhanced activation of the PI3K/AKT/mTOR pathway, as indicated by a 2.6-fold increase in p-RPS6 expression (p < 0.05), which functions as a surrogate marker for the activation of the PI3K/AKT/mTOR pathway.
Clinical study
The clinical application of wound dressings made from keratin protein is still in development; however, the literature does contain single studies that employ these dressings in clinical practice. As mentioned below, the introduction of keratin dressings has had a positive effect on the healing of wounds of various etiologies.
Than et al.49 investigated the use of keratin-based wound dressings in treating recalcitrant vascular wounds. This study presents 3 cases of patients with venous, arterial or mixed ulcers that had not healed with standard treatments, including compression therapy and traditional wound dressings. Each patient was treated with different formats of keratin dressings based on the wound’s characteristics (Keraderm gel – for dry wounds, matrix for moderately exuding wounds and Kerafoam for highly exuding wounds). Results showed successful healing in all three cases, with complete wound closure occurring within 10–30 weeks. The study suggests that keratin-based wound dressings promote epithelialization, improve wound healing rates, and provide a new option for managing chronic wounds.
In another study, Than et al. 50 investigated keratin hydrogel – Keragel® (Keraplast Technologies LLC, San Antonio, USA) in treating an 11-year-old girl suffering from recessive dystrophic epidermolysis bullosa (RDEB). They applied Keragel® to the neck daily for 3 months, which resulted in stronger, more resilient skin, reduced blistering and effective wound healing. The patient no longer needed secondary dressings, greatly improving comfort, appearance and quality of life.
In another study, Kirsner et al.51 used Keragel® (Keraplast Technologies, San Antonio, USA) to treat infant patient who experienced severe blistering, particularly on the hands and feet. Initially, the keratin-based dressing was applied only to one hand and one foot, while standard care was continued on the other hand and foot as a control. The study showed significant improvements in wound healing and skin strength, as well as reduced blister formation in the treated areas. Based on these promising results, treatment was extended to both hands and feet, leading to further improvements and eliminating the need for non-adherent silicone-based primary dressings.
Paulsen and Bygum52 evaluated the use of a keratin-based gel – Keragel® (Keraplast Technologies LLC, Christchurch, New Zealand) as an adjuvant therapy for recalcitrant pyoderma gangrenosum (PG) ulcers in a 62-year-old patient who was unresponsive to conventional systemic treatments. Despite administering corticosteroids, cyclosporine, methotrexate, and infliximab, the ulcers expanded and failed to epithelialize. Following the introduction of keratin gel (Keragel®) as the primary topical treatment, a significant reduction in ulcer size was observed within 9 days. Continued use of this dressing led to full healing after 6 months. The authors concluded that keratin gel may enhance epithelialization and promote faster wound closure, especially when systemic therapies alone are insufficient, suggesting its potential as a valuable adjunct in treating pyoderma gangrenosum ulcers.
Davidson et al.53 conducted a randomized control trial using a standard care dressing (alginate dressing (Algisite)) side by side with the experimental Keramatrix® (Keraplast) dressing for partial-thickness donor site wounds in 26 patients (“young” (≤50 y/o) and “old” (>50 y/o)). The results showed that keratin dressings significantly increased the rate of epithelialization in older patients (>50 years old), suggesting their clinical usefulness in cases where wound healing is delayed due to patient-related factors. However, in younger patients (≤50 years old), there was no significant difference in healing rates between keratin dressings and standard care.
Batzer et al.54 examined a solid keratin matrix (Keramatrix®) and a liquid keratin gel (Keragel™) from the Replicine™ Functional Keratin® range from Keraplast Technologies, in the treatment of 45 refractory wounds in 31 patients with mixed etiologies, including diabetic ulcers, venous leg ulcers and surgical wounds. Treatments included either a solid keratin matrix or a liquid keratin gel, depending on wound exudate levels. Results showed that 37/45 wounds (82%) either healed completely or reduced in size by at least 50%. Among them, 29 wounds (64%) were fully healed, and 8 wounds showed significant size reduction. They showed that keratin dressings effectively promote wound epithelialization. However, 15 wounds required antimicrobial treatment during the treatment, suggesting that keratin dressing treatment should be interrupted briefly and then restarted when wound infection occurs. Overall, the products were found to be satisfactory for patients and easy to use across a wide range of wound etiologies, but further research and clinical experience with these products is warranted.
Hughes et al.55 investigated the effectiveness of KeraStat® Cream (KeraNetics, Winston-Salem, USA), a keratin-based topical treatment, in managing radiation dermatitis (RD) in 24 patients undergoing radiotherapy (RT) for head and neck cancer (HNC). The patients were randomized to receive either KeraStat® Cream or standard care (SOC), with treatments applied at least twice daily during radiotherapy and for 1 month post-therapy. Adherence was high in both groups (KC 83% vs SOC 58% fully adherent), and while the cumulative incidence of grade 2+ RD did not differ significantly, the trial demonstrated good feasibility and patient compliance. KeraStat® Cream was well-tolerated and had good adherence, it did not significantly reduce the severity of radiation dermatitis compared to SOC. However, its anti-inflammatory properties and potential skin-protective effects warrant further investigation.
Conclusions
Keratin biomaterials represent a promising class of wound dressings that could transform routine treatment protocols for many types of wounds. Evidence from preclinical and clinical studies supports their safety, biocompatibility and ability to improve wound healing, even under diabetic conditions. However, while these results are promising, current research remains limited in scope and scale. The precise mechanisms by which keratin exerts its therapeutic effects are not yet fully understood. Moreover, although early clinical outcomes are positive, they are often derived from small-scale studies. Without large, multicenter randomized controlled trials, the translation of these results remains questionable. There is also a lack of comparative studies evaluating keratin dressings against standard treatments, which is essential for determining their relative clinical and economic value. Therefore, while keratin-based dressings demonstrate considerable potential, further rigorous investigation is essential. Future research should prioritize mechanistic studies, long-term safety evaluations and large-scale clinical trials. Through comprehensive research, keratin-based biomaterials have the potential to move beyond promising experimental studies and become a new clinical standard in wound treatment.