Abstract
Background. The two-drug regimen Kd-70 (carfilzomib at a dose of 70 mg/m2 with dexamethasone) is a recommended treatment option for patients with relapsed or refractory multiple myeloma, according to both American and European guidelines. However, aside from the A.R.R.O.W. trial, real-world data on its effectiveness remain limited.
Objectives. We aimed to assess the effectiveness of the two-drug regimen Kd-70 in real-world practice.
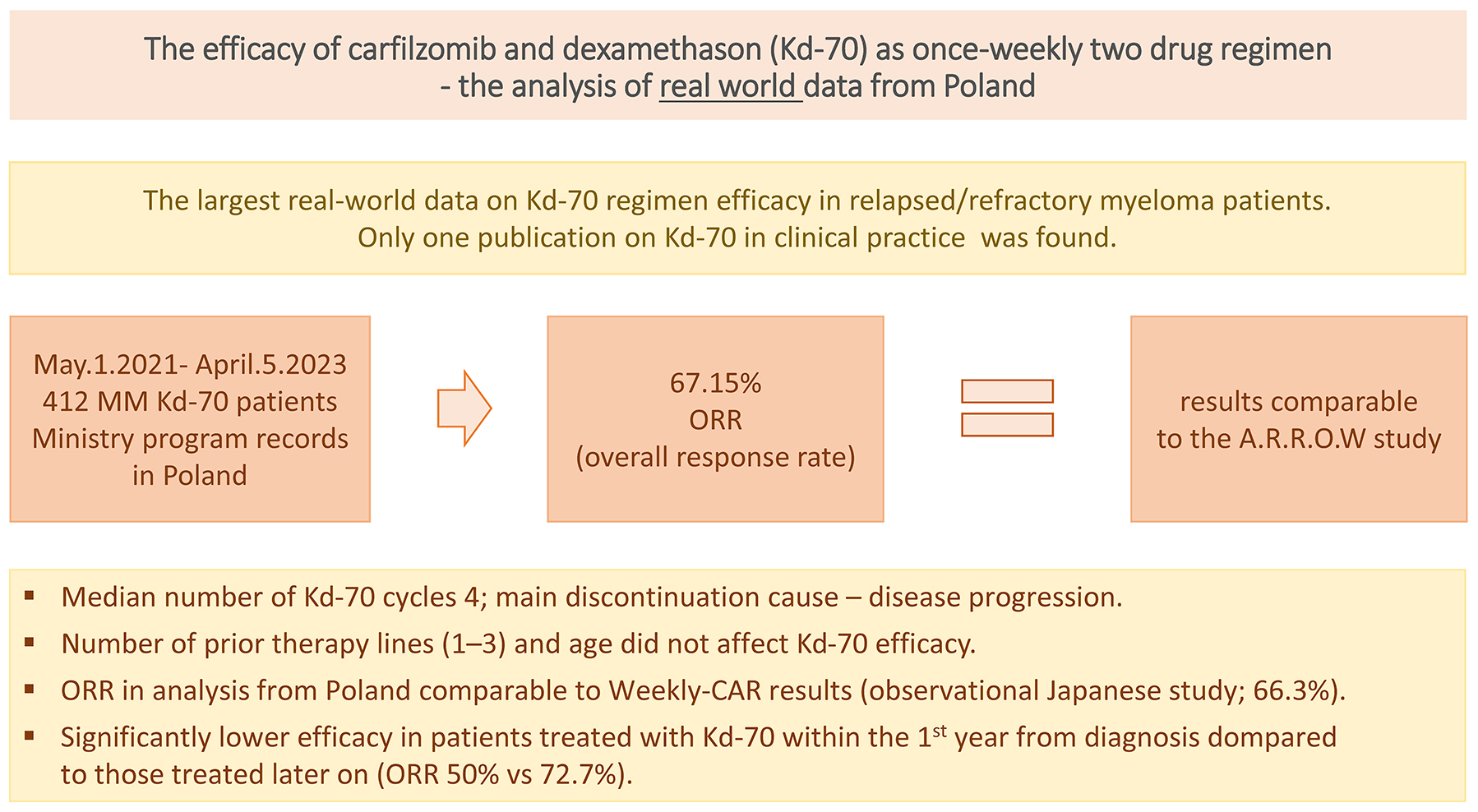
Materials and methods. We analyzed data from the Polish Ministry of Health registry, which included 412 patients treated with the Kd-70 regimen in Poland.
Results. The overall response rate (ORR) was 67.15%, comparable to the A.R.R.O.W. trial. However, the complete response (CR) rate (5.3%) and very good partial response (VGPR) rate (9.59%) were lower than those reported in the A.R.R.O.W. study. Notably, Kd-70 showed significantly lower efficacy in patients who required treatment for primary resistance or disease progression within the 1st year after diagnosis. In contrast, the number of prior treatment lines did not impact the regimen’s effectiveness.
Conclusions. In real-world clinical practice, the Kd-70 regimen demonstrated an ORR comparable to that observed in the A.R.R.O.W. trial. However, the CR and VGPR rates were lower. These findings underscore the need for further investigation into factors influencing treatment outcomes in this patient population.
Key words: myeloma, carfilzomib, Kd-70, real-word therapy data, relapsed/refractory myeloma
Background
The A.R.R.O.W. trial demonstrated that once-weekly dosing of carfilzomib (70 mg/m2) in combination with dexamethasone (Kd-70) is more effective than the traditional twice-weekly dosing of carfilzomib (27 mg/m2) in patients with relapsed and refractory plasma cell myeloma (RRMM).1 Specifically, progression-free survival (PFS) was significantly improved with the Kd-70 regimen (11.2 months vs 7.6 months, p = 0.003), while maintaining a similar safety profile. This registration study provides the evidence supporting the implementation of the Kd-70 regimen into clinical practice. According to the National Comprehensive Cancer Network (NCCN) guidelines, as well as the European Hematology Association – European Society for Medical Oncology (EHA–ESMO) recommendations, the Kd-70 schedule is a treatment option for selected previously treated myeloma patients.2, 3 Although 6 years have passed since the publication of the A.R.R.O.W. study results, to the best of our knowledge, only 1 publication has been released to date regarding the effectiveness of this two-drug regimen with carfilzomib at a 70 mg/m2 dosage in clinical practice.
Objectives
We aimed to assess the effectiveness of the two-drug regimen Kd-70 in real-world clinical practice, focusing on its performance in everyday clinical settings. We evaluated the response to Kd-70 and PDS in patients with RRMM, considering factors such as monoclonal protein type, age, the number of prior treatment lines, and the time since diagnosis.
Materials and methods
According to the Polish guidelines published between 2018 and 2023, the standard first-line therapy in Poland for transplant-eligible patients included bortezomib, thalidomide and dexamethasone (VTD). For patients ineligible for transplantation, the recommended regimen was melphalan, prednisone, and thalidomide (MPT).
Kd-70 was available during that period of time for 2nd-, 3rd- and 4th-line treatment under a ministry-regulated drug – access program. Lenalidomide with dexamethasone (LD) and daratumumab with bortezomib and dexamethasone (DVD) were also available through the same program.4, 5 In accordance with the guidelines of the Polish Ministry of Health, baseline patient demographics, clinical data and treatment outcomes for all patients treated within the drug programs had to be reported to the Ministry’s registers. We assumed that all patients met the eligibility criteria for the drug program that were in effect during the study period. These criteria included 1–3 prior therapy lines, no heart failure of New York Heart Association (NYHA) class III or IV, and no other cardiac contraindications to the use of carfilzomib, as well as sufficient bone marrow function, indicated by appropriate hematological parameters. We conducted an analysis of data from 412 consecutive patients reported in the Ministry of Health’s electronic records in Poland, covering the period from May 1, 2021, to April 5, 2023. The treatment protocol involved administering intravenous carfilzomib at 20 mg/m2 for the 1st dose in the first cycle, followed by 70 mg/m2 in subsequent weeks (on days 1, 8 and 15), in combination with oral dexamethasone (40 mg) once weekly, in 28-day cycles. Treatment was to be continued until evidence of disease progression. The primary endpoint of our study was the overall response rate (ORR). We analyzed various clinical data available in the Ministry’s records, including monoclonal protein type, age, gender, therapy line, and time since diagnosis, to identify response predictors. The current International Myeloma Working Group (IMWG) criteria were used to assess responses.
Statistical analyses
Data from all eligible patients were collected using a standardized, anonymized case report form. The collected variables included patient age, gender, M protein type, number of previous treatment lines, time from diagnosis to Kd-70 treatment, response to Kd-70 treatment, time to disease progression, and time to death. The distribution of continuous variables was assessed. Since age did not follow a normal distribution, as confirmed with the Shapiro–Wilk test (W = 0.9765, p < 0.001), it was reported as the median and interquartile range (IQR). Categorical variables were summarized as frequencies and percentages based on available data. Comparisons between groups based on response to Kd-70 treatment were performed using the χ2 test for categorical variables. Progression-free survival was defined as the time from the initiation of Kd-70 treatment to disease progression, death from any cause or the end of the observation period. Overall survival (OS) was defined as the time from the initiation of Kd-70 treatment to death from any cause or the end of the observation period. The probabilities of PFS and OS were estimated using the Kaplan–Meier method. All statistical analyses were performed using Statistica v. 13.3 (TIBCO Software Inc., Palo Alto, USA).
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. The study was approved by the Bioethics Committee of Wroclaw Medical University, Poland (approval No. KB 208/2023).
Results
Patients’ characteristics
The median age of the patients was 67 years (IQR: 61–72). There were 190 women (46.1%) and 222 men (53.9%). The majority of patients (74.8%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 1. Monoclonal protein characteristics were typical, with dominance of immunoglobulin G (IgG) (57.6%) in the heavy chain and kappa (67.5%) in the light chain. Selected laboratory and demographic characteristics are provided in Table 1. All patients were required to have adequate heart function, with an ejection fraction ≥40%, as patients with an ejection fraction <40% were excluded. Fifty-one percent of patients had received 1 prior line of treatment.
Response rate
Response to treatment was assessed in 344 patients, with 34 early deaths (ED) observed. Early deaths had occurred before assessment was conducted and were considered treatment failure, i.e., lack of response. In an additional 68 patients, the effectiveness of therapy was not assessed at the time of data collection. The ORR was 67.15%. Just under 15% of patients achieved a complete response (CR) or very good partial response (VGPR), approx. 52% met the criteria for a partial response (PR), and nearly 33% did not achieve a sufficient treatment effect (stable disease (SD), progressive disease (PD) or ED). Nearly 10% (34/344) of patients died before response assessment could be conducted. The best response achieved after a median of 2 cycles (range: 1–4) is shown in Figure 1. There was no difference in response rate based on patients’ age or the number of prior therapy lines (Table 2). The stratification into age groups, including the 40–49 age range, also did not reveal statistically significant differences in response to the Kd-70 regimen. However, our observations revealed a significant difference in ORR between patients who received Kd-70 within 1 year of diagnosis and those who received treatment later. Specifically, the ORR was 50% in the former group, compared to 72.7% in the latter group (p = 0.00003) (Table 3). Therapy was discontinued in 197 patients (47.8%) after a median of 4 cycles (range: 1–19 cycles) of Kd-70 due to various reasons (n = number of patients): no response (n = 120), death (n = 45) and toxicities (n = 22). In 6 patients, Kd-70 was not continued after autologous stem cell transplantation. In 4 patients, the reason for therapy termination was not provided. Among the 87 patients diagnosed ≤1 year before the introduction of Kd-70, therapy was discontinued in 58 (66.6%) cases, while in those diagnosed >1 year prior, therapy was discontinued in 42.7% (139/325) of cases.
Survival
The median follow-up time was 8 months (range: 1–23 months). The median progression-free survival (PFS) was 7.2 months (95% confidence interval (95% CI): 6.1–8.4), with a 1-year PFS rate of 35.1% (95% CI: 31.1–39.3) and a 1-year OS rate of 85.6% (95% CI: 62.0–95.6). A total of 45 deaths (10.9%) were reported during the follow-up period. The majority of these deaths, 34 in total, occurred before response assessment was conducted (ED). In most cases, no cause of death was reported, except for 5 patients in whom disease progression was noted. The death rate in patients treated with Kd-70 within 1 year of diagnosis was 20.7% (18/87), compared to 8.3% (27/325) in those diagnosed more than 1 year before Kd-70 implementation.
Discussion
The two-drug Kd-70 regimen (carfilzomib 70 mg/m2 and dexamethasone) was approved for use in Poland in May 2021 under the ministry-regulated access to the drug program. We analyzed the information available in the ministry’s records for all patients treated in the country (n = 412) from May 1, 2021, to April 5, 2023. Upon reviewing the collected data, we compared the obtained results with the findings of the registry study. Noteworthy distinctions emerged between the Polish Kd-70 population and the A.R.R.O.W. study. Our study group comprised patients treated with Kd-70 after 1–3 prior lines of therapy, whereas in the A.R.R.O.W. study, patients had received 2 or 3 prior lines of treatment, according to the inclusion criteria. Additionally, in our dataset, patients’ ECOG scores ranged from 0 to 2, while in the A.R.R.O.W. study, the inclusion criterion was ECOG scores of 0–1.6
Recently, an analysis of 114 cases of RRMM treated with Kd-70 in Japan, the “Weekly-CAR” study, was published. However, comparing our observations with the cohort described in this analysis is challenging due to differences in patient characteristics. In the mentioned study, more than 48% of the patients had previously received at least 4 lines of treatment.7
The ORR reported in our study, 67.15%, closely mirrored the outcomes documented in the A.R.R.O.W. study, where the ORR ranged between 61% and 65%, and in the Japanese publication, which reported an ORR of 66.3%. However, our Kd-70 population exhibited a lower percentage of CR and VGPR compared to the A.R.R.O.W. and “Weekly-CAR” studies. The therapeutic benefits of Kd-70 were evident regardless of age and the number of prior therapy lines, consistent with observations from the A.R.R.O.W. study.6
We were unable to find any publications on the clinical use of Kd-70 beyond the “Weekly-CAR” project. Therefore, we briefly compare our results with studies that exclusively or primarily involve earlier versions of the Kd regimen, including the twice-weekly administration of carfilzomib.
In a prospective observational study (n = 273; carfilzomib at a dose of 20/56 mg/m2 weekly), the ORR was 63% in patients who had previously received an average of 2 or 3 lines of treatment. The mortality rate ranged from 7% in groups without lenalidomide resistance to 9% in those with lenalidomide resistance.8
Comparable ORR values were reported in a study by Terpos et al.,9 with an ORR of 62.1% (n = 93; carfilzomib at a dose of 20/56 mg/m2 weekly), and by Onda et al., with an ORR of 62% (n = 41 for Kd-56 and n = 9 for Kd-70).10
In another observational study (n = 103; carfilzomib at a dose of 20/56 mg/m2 weekly), the ORR was 54.7%. Notably, nearly 36% of patients in this cohort were treated with this regimen as the 5th or later line of therapy.11
An assessment of the safety profile based on the available data was not possible. We found that Kd-70 therapy was discontinued after an average of 4 cycles in the Polish patient population. The primary reason for discontinuation was lack of efficacy, followed by the occurrence of adverse effects. This is consistent with observations from the “Weekly-CAR” project.7
Our analysis revealed a significant distinction among patients treated with Kd-70 within the 1st year of diagnosis. This subgroup demonstrated significantly poorer outcomes compared to those who received Kd-70 at a later stage. These outcomes included a lower response rate, higher mortality and more frequent discontinuation of Kd-70 therapy. This disparity suggests a potential association between treatment refractoriness to Kd-70 in patients treated within 1 year of diagnosis and the presence of primary resistance, characterized by a more aggressive clinical course.
Limitations
This study has several limitations, primarily stemming from its retrospective design and the limited clinical data available in the ministry’s drug program registries. Notably, the absence of information on patients’ genetic profiles and the specific types of adverse events limits the ability to conduct a more comprehensive and detailed analysis. Nevertheless, it is important to note that these data provide valuable insights derived from real-world practice involving the two-drug Kd-70 regimen in a substantial population of patients with relapsed and refractory multiple myeloma.
Conclusions
In real-world practice, the Kd-70 regimen yielded a comparable ORR to the results observed in the A.R.R.O.W. trial, but with a lower rate of CR and VGPR. Notably, treatment outcomes with the Kd-70 regimen during the 1st year following diagnosis were markedly inferior compared to those in patients with a more extended treatment history. This discrepancy may be attributed to primary resistance and a more aggressive disease profile within this particular subgroup.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.

















.jpg)