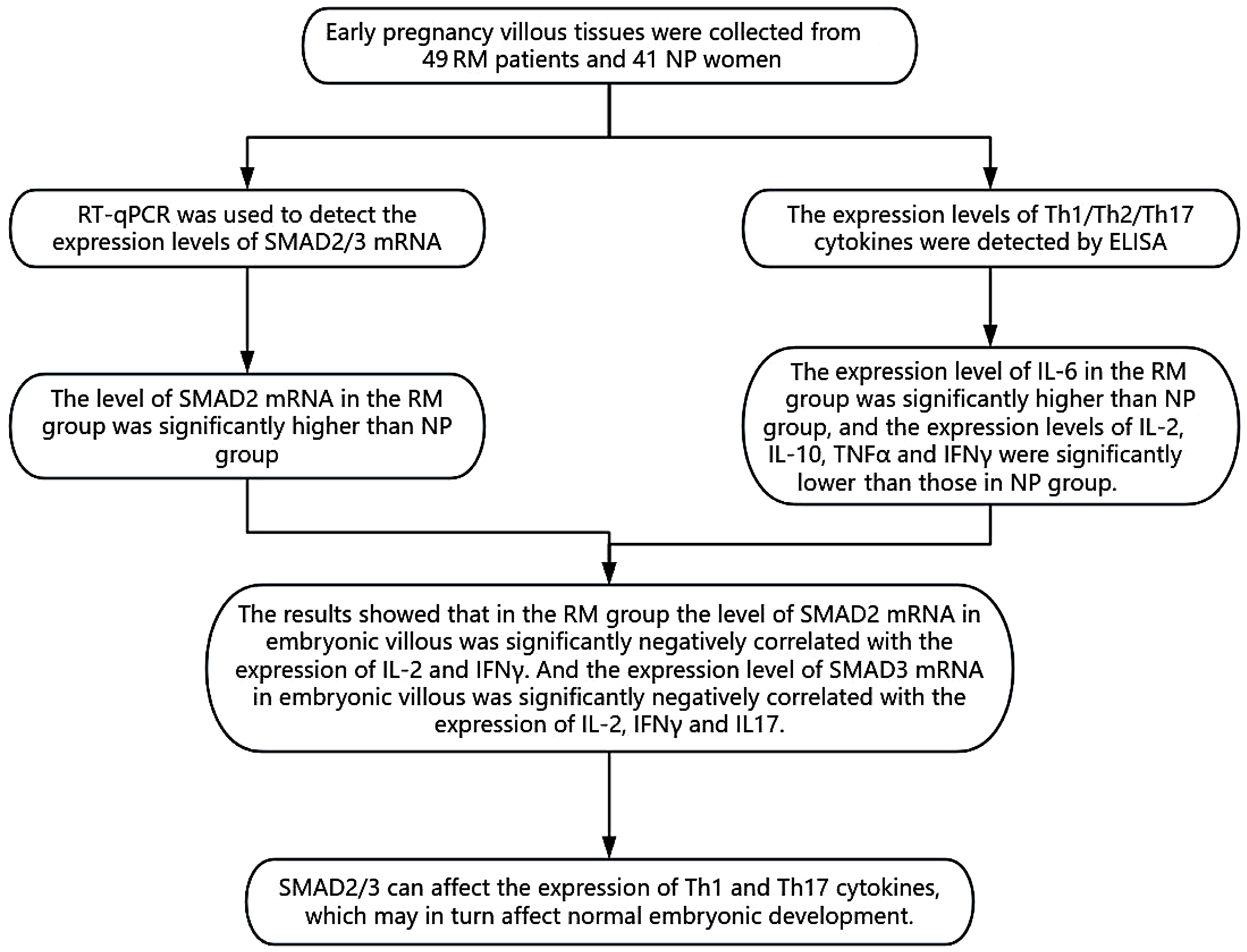
Abstract
Background. Recurrent miscarriage (RM), the loss of 2 or more consecutive pregnancies before 28 weeks’ gestation, has become increasingly common in recent years, imposing significant physical and psychological burdens on affected women. Despite comprehensive evaluation, approx. 40–50% of RM cases remain unexplained and are therefore classified as idiopathic.
Objectives. This study aimed to investigate the expression of SMAD2 and SMAD3 and characterize Th1, Th2 and Th17 cytokine profiles in placental villous tissues from women with idiopathic RM.
Materials and methods. Forty-nine women with idiopathic RM in early pregnancy and 41 gestational age-matched women with normal pregnancies (NP) were recruited at Shanxi Maternal and Child Health Hospital (Taiyuan, China) . Following informed consent, placental villous tissues were obtained via ultrasound-guided vacuum aspiration, rinsed in saline and stored at –80°C. Total RNA was extracted from each sample, and SMAD2 and SMAD3 mRNA levels were quantified using real-time quantitative PCR (qPCR) using the 2–ΔΔCq method. Parallelly, tissue homogenates were assayed with enzyme-linked immunosorbent assay (ELISA) for Th1 cytokines (interleukin (IL)-2, intereron gamma (IFN-γ), tumor necrosis factor alpha (TNF-α)), Th2 cytokine (IL-10), and Th17 cytokines (IL-6, IL-17). Spearman’s rank correlation was used to evaluate associations between SMAD2/3 expression and cytokine concentrations. All statistical analyses were performed in IBM SPSS v. 27.0, with two-tailed p < 0.05 denoting significance.
Results. The qPCR analysis demonstrated that SMAD2 mRNA levels in villous tissues were significantly higher in the RM group than in NP controls (p < 0.05). Consistent with this, ELISA revealed a marked increase in IL-6 concentration (p < 0.05) alongside significant reductions in IL-2, IL-10, TNF-α, and IFN-γ levels in RM samples vs NP (all p < 0.05). Spearman correlation analysis showed that SMAD2 expression was inversely correlated with IFN-γ (ρ < 0, p < 0.05), while SMAD3 expression was negatively associated with both IL-2 and IFN-γ levels (ρ < 0, p < 0.05).
Conclusions. SMAD2/3 can affect the expression of Th1 and Th17 cytokines, which may in turn affect normal embryonic development.
Key words: cytokines, embryo, SMAD2, SMAD3, idiopathic recurrent miscarriage
Background
Recurrent miscarriage (RM) is defined as the consecutive loss of 2 or more pregnancies in the same woman before 28 weeks of gestation. Patients with RM face an approx. 40% risk of embryo loss in subsequent pregnancies,1 a burden that profoundly impacts reproductive health. The etiology of RM is multifactorial, encompassing infectious agents, uterine anatomical abnormalities, immune dysregulation, endocrine disorders, and chromosomal anomalies. Nevertheless, despite comprehensive evaluation, a substantial proportion of RM cases remain unexplained, highlighting a critical and active area of clinical research.
Cases in which no cause can be identified are classified as idiopathic RM. Immune regulation at the maternal–fetal interface is critical for pregnancy maintenance. At the maternal interface, the decidua not only supports embryo implantation and development but also mediates maternal immune tolerance.2, 3 At the fetal interface, trophoblast cells adhere to and invade the maternal endometrium to secure nutritional exchange and collaborate with decidual immune cells to establish a specialized immune microenvironment4. Recurrent miscarriage has been associated with disruptions in this maternal–fetal immune balance5, most notably an altered Th1/Th2 cytokine ratio at the interface. Th17 cells are integral to maintaining immune equilibrium at the maternal–fetal interface and supporting healthy pregnancies. Disruption of this balance can trigger maternal immune rejection of the embryo, resulting in miscarriage.6 In early gestation, a Th1-dominant response-mediated by cytokines such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) drives the inflammatory milieu necessary for successful implantation. As pregnancy advances, Th2 cytokines (e.g., interleukin (IL)-4 and IL-10) increasingly promote maternal–fetal tolerance, shielding the fetus from immune attack. Whether the Th1/Th2 balance further shifts toward Th2 during the second and third trimesters to facilitate labor remains controversial,7 underscoring the need for additional research into cytokine dynamics throughout gestation. Meanwhile, SMAD2 and SMAD3-key transcriptional mediators of tumor growth factor beta (TGF-β) signaling, govern critical processes in cell differentiation, immune regulation and embryonic development.8, 9
SMAD2 and SMAD3 are also key regulators of Th1, Th2 and Th17 cell proliferation and differentiation.10, 11 Downregulation of the TGF-β–mTOR–hypoxia-inducible factor 1 alpha (HIF-1α) pathway has been shown to increase cytotoxicity, reduce apoptosis, and alter cytokine secretion by decidual natural killer (dNK) cells, thereby contributing to the pathogenesis of RM.12 Inhibition of SMAD2/3 similarly impairs dNK cell function and promotes pregnancy-related disorders via modulation of this pathway. In animal models, icaritin (ICT) has been demonstrated to decrease fetal loss by restoring Th17-mediated immune balance through activation of the TGF-β–SMAD2/3 axis.13 However, most studies to date have focused on model organisms, and data on SMAD2/3 expression in human RM patients remain scarce. Moreover, investigations of immune mechanisms in RM have largely centered on the maternal–fetal interface, with relatively little exploration of the fetal interface. Our previous work revealed significant alterations in TGF-β/BMP pathway gene expression in villous tissues from RM compared to normal pregnancy groups.14
Objectives
This study aims to further elucidate the role of SMAD2/3 in RM by conducting an in-depth investigation of embryonic villous tissue from RM patients, and explore the role of SMAD2/3 in immune response through analyzing its relationship with cytokines. In addition, immunological studies of fetal villous tissue will provide new evidence for understanding the mechanism of RM.
Materials and methods
Research subjects
Between July 2023 and October 2024, we enrolled 49 women with RM who presented to the Shanxi Maternal and Child Health Hospital (Shanxi Children’s Hospital; Taiyuan, China). All had experienced at least 2 consecutive embryonic losses before 12 weeks’ gestation, confirmed with transvaginal ultrasound. Detailed histories, including thromboembolic disorders and prior pregnancy losses, were obtained, and classical RM risk factors (parental chromosomal abnormalities, uterine anatomical defects, infectious diseases, luteal phase insufficiency, diabetes mellitus, thyroid dysfunction, and hyperprolactinemia) were systematically excluded. As a control group, we recruited 41 women with uncomplicated, clinically normal pregnancies (NP) who were undergoing elective termination.
All participants were screened for established risk factors for pregnancy loss. After obtaining informed consent, both RM patients and NP controls underwent ultrasound-guided curettage abortion, and villous tissue specimens were collected. Samples were accepted only if they met both of the following criteria: 1) absence of uterine bleeding or cramping, with ultrasound confirmation of an empty gestational sac or an embryo without cardiac activity; and 2) retrieval of a fresh specimen, free of blood clots, via vacuum aspiration. Collected tissues were rinsed in sterile saline and snap-frozen at –80°C. This study protocol was reviewed and approved by the Ethics Committee of Shanxi Children’s Hospital (approval No. IRB-KYSB-2023-030). All experimental participants signed the written informed consent form.
Experimental methods
The expression of SMAD2/3 mRNA transcript levels in RM and NP samples detected with qPCR
Total RNA were extracted from 10–20 mg villous tissue of RM and NP samples according to the instructions of RNAprep Pure Tissue Kit (Tiangen Biotech, Bejing, China). The concentration and purity of RNA were quantified with NanoDrop microspectrophotometer (Thermo Fisher Scientific, Waltham, USA). RNA samples were examined using a Bio-Rad (Hercules, USA) electrophoresis apparatus and RNA integrity was assessed by observing 28S and 18S RNAs strips. Then 3 μg total RNA was reverse transcribed into cDNA using Universal RT-PCR Kit (M-MLV) (Solarbio, Beijing, China). Reverse transcription reactions were carried out in a total volume of 20 μL containing 2 μg total RNA, 2 μL Oligo(dT)16, Supplement RNase-free ddH2O to 14.5 μL. After initial denaturation at 70°C for 5 min, the mixture was immediately cooled on ice for 2 min and briefly centrifuged. Subsequently, 4 μL 5 × M-MLV Buffer, 1 μL dNTPs, 0.5 μl RNasin, and 1 μL M-MLV were added. The reaction proceeded at 42°C in a PCR instrument for 60 min, followed by enzyme inactivation at at 95°C for 5 min. The resulting cDNA was diluted to 50 μL with RNase-free ddH2O. Real-time qualitative polymerase chain reaction (qPCR) was performed using the SYBR Green qPCR Master Mix (Bioss, Beijing, China) kit, with Human ACTB Endogenous Reference Genes Primers (Sangon Biotech, Shanghai, China) used as internal reference. Thermal cycling conditions comprised: initial denaturation at 95°C for 30 s; 40 cycles of 95°C for 3 s and 60°C for 30 s; followed by melt curve analysis (65–95°C, 0.5°C increments). All reactions were performed on a CFX Real-Time PCR Detection System (Bio-Rad) thermocycler. Primers for SMAD2 and SMAD3 were designed using the Primer-BLAST tool of the National Center for Biotechnology Information (NCBI) database (Bethesda, USA). Data concerning primers used are shown in Table 1. The relative expression of samples were calculated using Bio-Rad CFX Manager v. 3.0.
Detection of Th1/Th2/Th17 cytokine expression levels in the villous of patients in the RM and NP samples using ELISA
Total protein was extracted from ~50 mg of villous tissue using the Total Protein Extraction Kit (Solarbio, Beijing, China) per the manufacturer’s instructions. Protein concentrations were determined using bicinchoninic acid (BCA) assay (Tiangen Biotech), and equal amounts of protein, normalized to these measurements, were applied to 96-well plates pre-coated with the appropriate capture antibodies. Cytokine levels (IL-2, IFN-γ, IL-6, TNF-α, IL-17, and IL-10) were quantified using enzyme-linked immunosorbent assay (ELISA) kits (Bioss Antibodies, Beijing, China; cat. No. bsk11002, bsk11013, bsk11007, bsk11014, bsk11028, and bsk11010, respectively). Absorbance at 450 nm was read on a Diatek microplate reader (Diatek, Wuxi, China), and sample concentrations were calculated against standard curves using WPS Office (Kingsoft, Beijing, China).
Statistical analyses
Statistical analyses were performed in IBM SPSS v. 27.0 (IBM Corp., Armonk, USA). Data distribution was assessed using the Shapiro–Wilk test. For variables that followed a normal distribution, group comparisons were made with independent-samples t-tests and are reported as mean ± standard deviation (SD). Non-normally distributed data were compared using the Mann–Whitney U test and are presented as median with interquartile range (IQR, 25–75th percentiles). Associations between SMAD2/3 expression and cytokine levels were evaluated using Spearman’s rank correlation. Multiple comparisons were corrected using the Bonferroni method: Each p-value was multiplied by the number of cytokines tested (k = 6) and truncated to a maximum of 1.0, with significance defined as an adjusted p < 0.05. SMAD2 and SMAD3 relative expression levels were quantified using the 2–ΔΔCq method, and differences were considered statistically significant at p < 0.05.
Results
Clinical information
There were no significant differences in either maternal age or gestational age between the RM (recurrent miscarriage) and NP (normal pregnancy) groups (Table 2).
qPCR results
The qPCR analysis revealed that SMAD2 expression was significantly upregulated in the RM group compared to NP controls, whereas SMAD3 expression was markedly reduced in the RM group (Table 3).
Th1, Th2 and Th17 cytokine expression levels
In placental villous tissues, IL-2, TNF-α, IFN-γ, and IL-10 concentrations were significantly reduced in the RM group compared to NP controls (p < 0.05), whereas IL-6 levels were significantly elevated (p < 0.05). Although IL-17 appeared lower in RM samples, this difference did not reach statistical significance (p > 0.05) (Table 4).
Cytokines ratios in placental villous tissue
The ratios of IL-2/IL-10, IFN-γ/IL-10 and IL-17/IL-10 were significantly higher in the RM group compared to the NP group (p < 0.05), while the TNF-α/IL-10 ratio was also elevated but did not reach statistical significance (p > 0.05) (Table 5).
Correlation analysis of SMAD2/3 and Th1/Th2/Th17 cytokine levels in RM group
Spearman’s correlation analysis demonstrated that SMAD2 expression was significantly negatively correlated with IFN-γ levels, while SMAD3 expression was significantly negatively correlated with both IL-2 and IFN-γ levels (Table 6).
Discussion
Recurrent miscarriage is among the most common complications affecting women of reproductive age.1 Women with a history of RM face an elevated risk of miscarriage in subsequent pregnancies and are more likely to experience other obstetric complications, such as fetal growth restriction and stillbirth. The pathogenesis of RM is closely linked to dysregulation of the maternal–fetal immune interface, highlighting the critical role of immune factors in maintaining a successful pregnancy.
SMAD2/3 are intracellular kinase substrates of TGF-β receptors, facilitating intracellular signal transduction of TGF-β. They regulate the expression of downstream target genes by interacting with other transcription factors, thereby affecting the initiation and development of various biological processes such as cell growth, proliferation, apoptosis, migration, adhesion, and differentiation.15 Although SMAD2/3 have been reported to play critical roles in early embryonic development, the relationship between SMAD2/3 in embryonic villous tissue and miscarriage remains unexplored. SMAD2/3 influence the trajectory of the embryo by directly transcribing target genes via Nodal/Activin-mediated TGF-β signaling cascades.16, 17 In addition, SMAD2 plays an important role in establishing embryonic axial patterns and specifying deterministic endoderm. Mouse embryos with SMAD2 deficiency are unable to undergo normal embryo transformation and establish the anterior posterior axis, resulting in embryonic mortality.18, 19 SMAD2 is also vital for embryo elongation and mesoderm induction. A deficiency in SMAD2 prevents mouse embryos from undergoing these crucial processes before the embryonic stage.20 Meanwhile, the synergistic activity of SMAD2/3 regulate mesoderm formation and patterning as well as embryonic morphogenesis in mice. While SMAD3 provides essential signals during early implantation, dual absence of SMAD2/3 completely blocks mesoderm formation and embryonic development.21 In human embryonic stem cells (hESCs), SMAD2 and SMAD3 coordinate the maintenance of pluripotency and the onset of differentiation by modulating distinct transcriptional networks and engaging with chromatin-modifying complexes.9 In our study, SMAD2 was significantly higher in RM than NP. The major role of SMAD2 in early embryonic development is to control epigenetic modifications through the activin/SMAD2 signaling pathway and to cooperate with the Wnt/β-catenin signaling to regulate mesoderm gene expression.22, 23 Therefore, we speculate that high expression of SMAD2 may affect mesoderm development, but further validation is needed.
The roles of Th1, Th2 and Th17 cells in embryonic development involve the production and regulation of various essential immune system cytokines. Th1 cells mainly produce cytokines such as il-2, IFN-γ and TNF-α, promoting cell-mediated immune responses.24 Interleukin 10 is mainly secreted by Th2 cells, but can also be produced by regulatory T cells (Treg), Th1 cells and Th17 cells. Th2 cytokines contribute to promoting immune tolerance at the mother-fetal interface and ensuring the maintenance of normal pregnancy.25 While IL-6 is commonly recognized as a proinflammatory cytokine, it also exhibits anti-inflammatory properties.26 This dual functionality allows IL-6 to play a nuanced and intricate role in maintaining inflammatory homeostasis. The number of Th17 cells at the mother–fetal interface significantly increases during early pregnancy, potentially playing a crucial role in the proliferation and invasion of trophoblastic cells, as well as subsequent placental formation. In addition, Th17 cells regulate the biological activity of trophoblast cells by secreting cytokines, especially IL-17 and play an important role in placental development in early pregnancy.27 In this period, TNF-α supports the pregnancy process by regulating hormone synthesis, placental structure and embryonic development. The increased expression level of TNF-α contributes to the maturation of the placenta and the normal development of the embryo.28 IFN-γ can induce trophoblast apoptosis and MHC II antigen expression, thereby protecting placental function.29 Furthermore, IFN-γ helps to inhibit immune rejection and protect the embryo by regulating the polarization of Treg cells and Th17 cells.30 Interleukin 2 can stimulate the proliferation and function of regulatory Tregs, regulate the activity of immune cells, promote maternal immune tolerance to the fetus, and contribute to placental stability and normal fetal development.31, 32
Our study demonstrated that women in the RM group exhibited significantly lower levels of IL-2, TNF-α, IFN-γ, and IL-10 compared to the NP cohort, a finding that parallels the majority of previous reports. The level of IL-6 was significantly increased. Beyond its role in inflammation, IL-6 contributes to embryo implantation, placental development and pregnancy maintenance.27 Previous research has suggested inadequate expression or secretion of IL-6 at the fetal–maternal interface in patients with RM.33 However, increased IL-6 expression in the decidua has been reported in recent studies.34, 35 Elevated levels of IL-6in decidual tissue indicate increased inflammation at the fetus–maternal interface, which may potentially endanger embryo implantation and pregnancy. Therefore, both low and high levels of IL-6 can disrupt the inflammatory network at the fetus-maternal interface, thereby affecting pregnancy outcomes. Although studies on the expression of IL-6 in villous tissues are limited, our preceding study found that the expression of IL-6 was significantly higher in the villous tissues of RM patients compared to NP group. This finding was verified in the present study.14 Given that IL-6 can attenuate maternal immune rejection of the fetus, we hypothesized that persistently elevated IL-6 levels in RM reflect ongoing alloimmune reactions at the fetal interface; as these responses intensify, IL-6 expression rises further, ultimately contributing to pregnancy loss.36 Analysis of the four indicators IL-2/IL-10, TNF-α/IL-10, IFN-γ/IL-10 and IL-7/IL-10 revealed higher levels in the RM group, mainly because the expression of IL-10 in the RM group was much lower than that in the NP group. Interleukin 10 maintains a homeostasis between mother and embryo by suppressing inflammation and regulating the immune system.37 For example,IL-10 protects embryo from inflammation-mediated damage by inhibiting TNF-α production in macrophages.24 Moreover, IL-10 inhibits invasion of placental interstitial cells by inhibiting the expression of matrix metalloproteinase 9 (MMP-9), which is crucial for embryo implantation and growth in the uterus.38 In early-pregnancy decidual tissues from women with RM, concentrations of IL-17, IL-6 and TNF-α were significantly elevated,33, 39, 40 whereas IL-10 expression was markedly reduced.41 Notably, IFN-γ levels did not differ between RM and control groups.42 These observations contrast with the cytokine profiles seen in villous tissues, highlighting distinct maternal vs fetal immune signatures during the first trimester.
SMAD2 and SMAD3 are essential downstream effectors of the TGF-β/SMAD signaling pathway, and multiple studies have established TGF-β as a pivotal regulator of T helper cell differentiation.43, 44 Takimoto et al. found that Smad2/3-dependent TGFβ inhibited Th1 and Th2 differentiation.45 In Nile tilapia (Oreochromis niloticus), TGF-β1 has been shown to activate the canonical TGF-β/SMAD signaling pathway.46 This leads to subsequent phosphorylation and nuclear translocation of SMAD2/3. Furthermore, SMAD3 interacts with various transcription partners to inhibit the transcription of cytokines IL-2 and IFN-γ.47 Wang also found that SMAD3 inhibits Th17 cell differentiation by interfering with the transcriptional activity of RORγt.48 In our research, SMAD2 was significantly negatively correlated with IFN-γ. SMAD3 was significantly negatively correlated with IL-2 and IFN-γ. These findings suggest that SMAD2/3 may inhibit the production of Th1 and Th2 cytokines.
Limitations
This single-center study was conducted exclusively in Shanxi Province, and may therefore have limited generalizability. We confined our analysis to early pregnancy to capture the critical window of embryo implantation and immune-microenvironment establishment; however, extending investigations into the second and third trimesters would yield valuable insights into immune dynamics throughout gestation. Additionally, we did not examine pathological correlations – an important avenue for future research given the complexity of immune–tissue interactions. Finally, our modest sample size precluded the development of robust statistical models, underscoring the need for larger, multicenter cohorts.
Conclusions
SMAD2/3 can affect the expression of Th1 and Th17 cytokines to alter the immune environment of embryonic villous tissue, which may in turn affect normal embryonic development. SMAD2 and SMAD3 represent promising novel biomarkers and therapeutic targets for the diagnosis and management of idiopathic recurrent miscarriage.
Data availability statement
The datasets supporting the findings of the current study are openly available in the Zenodo repository at https://doi.org/10.5281/zenodo.15234132.
Consent for publication
Not applicable.
Use of AI and AI-assisted technologies
Not applicable.