Abstract
Background. The number of infants born via cesarean section (CS) is increasing globally due to medical and cultural reasons.
Objectives. This study aimed to determine the effect of the mode of delivery on early lung aeration in newborns using electrical impedance tomography (EIT).
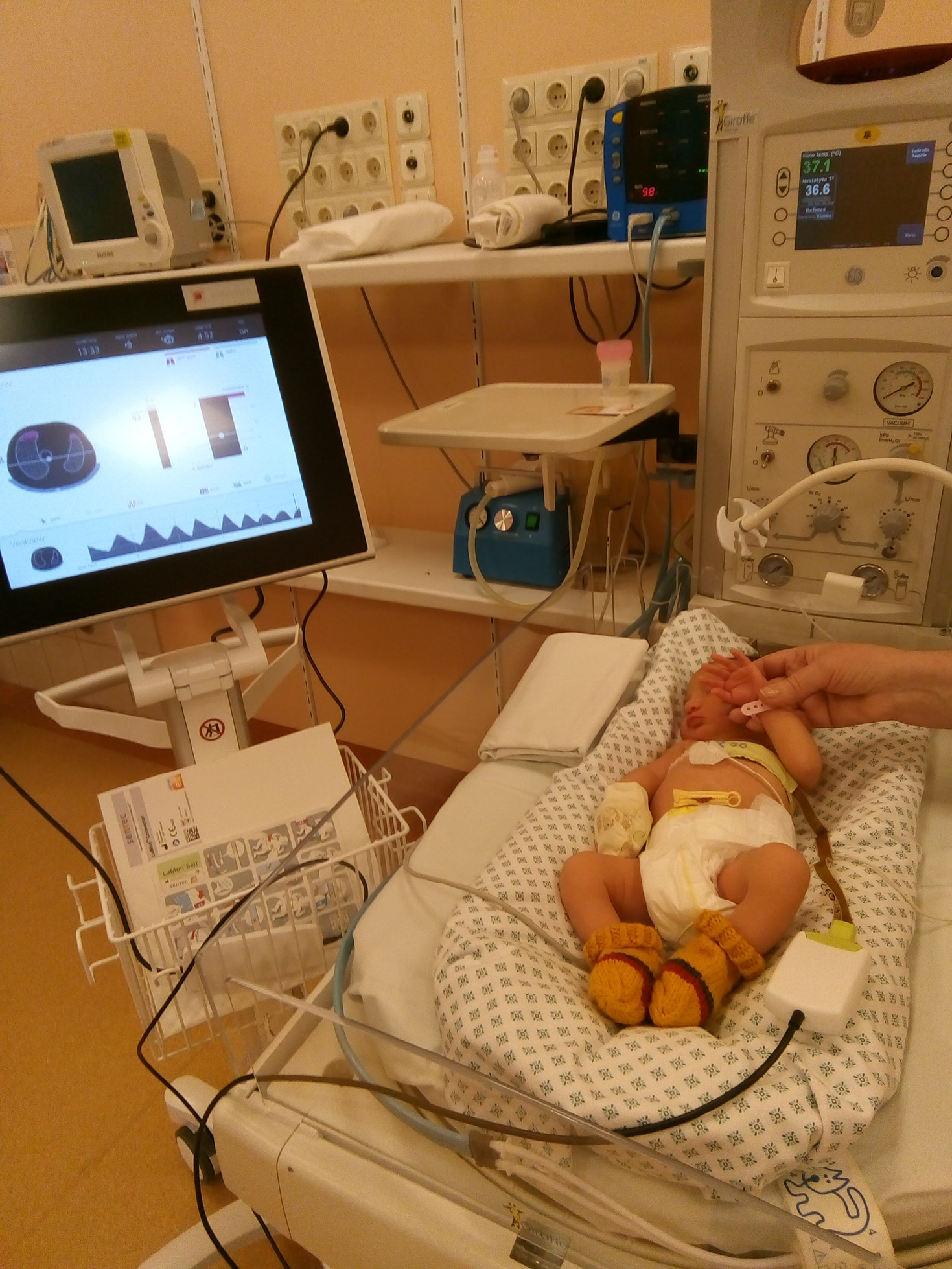
Materials and methods. The case-control study was conducted from December 2020 to April 2021. It included 32 term neonates delivered by CS and 20 term neonates delivered by normal vaginal delivery (NVD) as controls. Electrical impedance tomography examinations were performed with a Swisstom BB2 device with NEO SensorBelt and 32 integrated electrodes at 47.68 Hz. Three data recordings were conducted within 30, 60 and 90 min (mean times: 13, 62 and 93 min, respectively) after the birth.
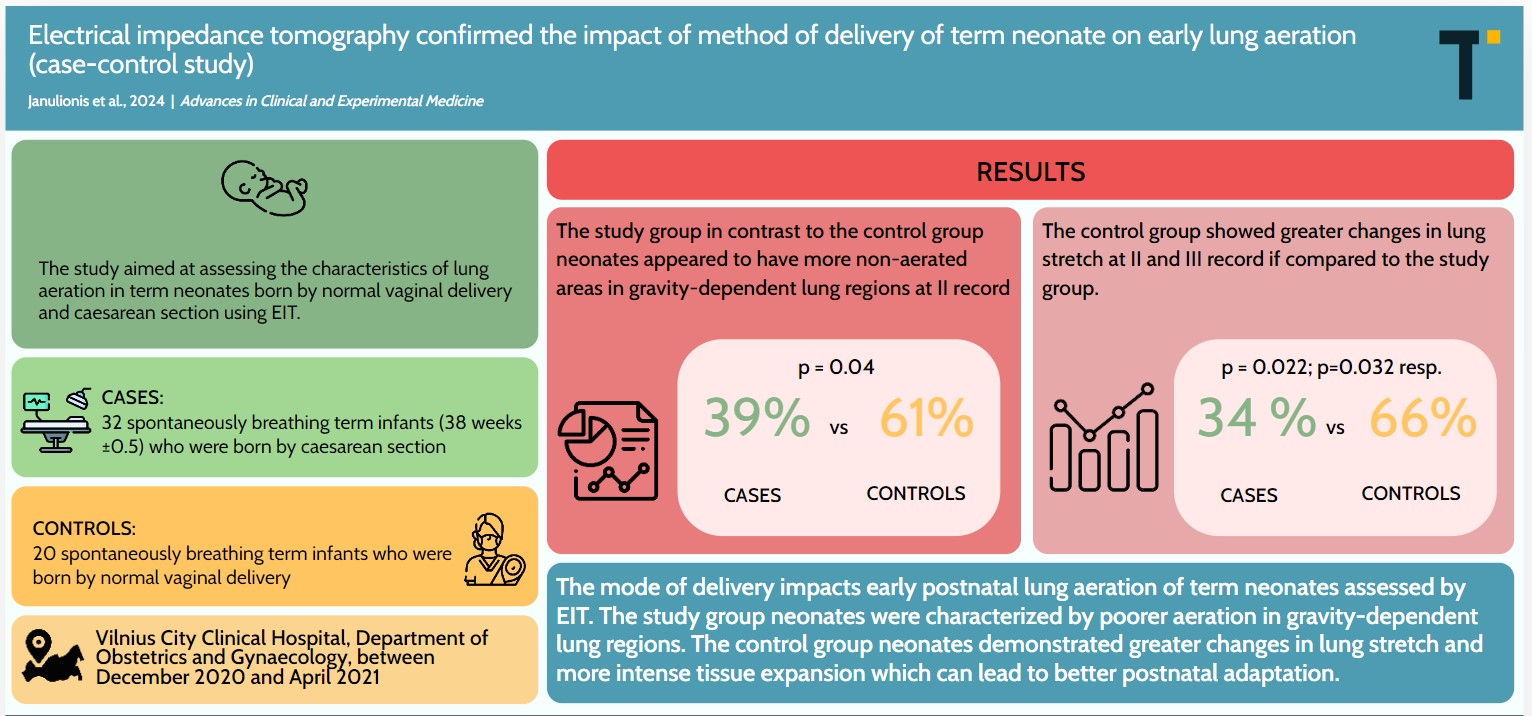
Results. Cesarean section neonates, compared to those delivered by NVD, had greater non-aerated areas in gravity-dependent lung regions at the 2nd recording (p = 0.04). The CS group showed lower changes in lung stretch at the 2nd and 3rd recording compared to the NVD group (p = 0.022 and p = 0.032, respectively). In the study group, lung regions with the lowest stretch (10–20%, 20–30% and 30–40%) corresponded with increased total lung capacities 1 h after birth compared to the control group (p = 0.024, p = 0.004 and p = 0.044, respectively). Measurements from the 1st and 3rd EIT recording showed a greater distribution of tidal volume (TV) in the right lung toward the central regions among CS neonates compared to NVD neonates, whereas NVD neonates showed increased distribution of TV toward the central-ventral regions of the right lung immediately after birth.
Conclusions. The mode of delivery significantly affects early postnatal lung aeration in term neonates assessed using EIT. Caesarean section neonates were characterized by poorer aeration in gravity-dependent lung regions, whereas NVD neonates demonstrated greater changes in lung stretch and more intense tissue expansion, potentially leading to better postnatal adaptation.
Key words: cesarean section, normal vaginal delivery, electrical impedance tomography, lung aeration, term neonates
Background
The transition from the intrauterine to the extrauterine environment poses significant challenges for neonates, primarily due to fundamental changes in their breathing and circulation. Fetal lungs are known to be filled with fluid, and thus, the aeration of the lungs with air is one of the most important physiological changes that occur immediately after birth. However, it remains unclear exactly how and when the fluid is completely removed from the lungs. Several key mechanisms and stages of fluid clearance from the lungs have been identified, starting during fetal development and continuing through labor and the first hours after birth.1 It is also known that both gestational age and the method of birth influence lung fluid clearance and aeration.1, 2 However, it is uncertain whether all lung regions achieve uniform aeration and synchronization post-birth, or if differences in lung aeration exist depending on the mode of delivery and other factors.
A variety of methods and techniques are available for assessing lung aeration in neonates after birth. These methods have been summarized in a recently published systematic review.3 However, some methods are indirect (such as respiratory monitoring and capnography), while others involve ionizing radiation (such as chest radiography), and static, one-dimensional lung imaging. Additionally, lung ultrasonography reflects the overall fluid–air ratio in the lung rather than providing clear identification of the aeration of individual lung segments. In recent years, noninvasive techniques such as respiratory inductive plethysmography or electrical impedance tomography (EIT) have been increasingly used to assess the dynamic changes in lung aeration in neonates.4 Earlier studies from Lithuania have highlighted the utility of EIT in both preterm and term neonates.5, 6, 7, 8
Objectives
The study aimed to assess the characteristics of lung aeration in term neonates born via NVD and cesarean section (CS) using EIT.
Materials and methods
Study participants
Our case-control study included 52 spontaneously breathing term neonates born at the Department of Obstetrics and Gynecology of the Vilnius City Clinical Hospital (Lithuania), between December 2020 and April 2021. Among these, 20 were born via NVD (control group) and 32 infants (38 weeks ±0.5) were delivered by CS (study group). This included 28 elective CS and 4 emergency surgeries. All emergency CS were conducted due to abnormal fetal heart rate patterns. None of the infants required assistance for adaptation in the delivery room. Neonates meeting the inclusion criteria were randomly selected.
The study was approved by the Vilnius Regional Biomedical Research Ethics Committee (approval No. 1287 issued on November 24, 2021), and written informed consent was obtained from the parents of all newborns. The main characteristics of the newborns are presented in Table 1.
Data collection utilized the Electrical Impedance Tomography device (Swisstom AG, Landquart, Switzerland). A NEO SensorBelt equipped with integrated 32 electrodes was positioned circumferentially around the thorax at the nipple level, operating at a sample rate of 47.68 Hz.
Three data recording sessions were conducted on the day of birth for each neonate. Data were collected at 3 intervals: immediately after birth (0 min), 60 min after birth and 90 min after birth. All recordings were conducted with the infants in the supine position. For breastfeeding sessions, measurements were delayed to avoid disrupting mother–infant bonding. Similarly, for any procedures such as blood draws, heel pricks or routine nursing care, measurements were postponed from the scheduled time frames unless the delay exceeded 30 min beyond the designated time frame. Each EIT recording session lasted a minimum of 5 min. All studied infants were spontaneously breathing. Figure 1 shows the process of data recording.
Data analysis was conducted using Ibex 1.4 software (https://www.sentec.com/fileadmin/documents/_EIT_documents/ibeX_Product_Information.pdf). The impedance signal was processed using a respiratory rate filtering function. The following EIT parameters were compared between the 2 groups of study participants:
1. Center of ventilation (CoV), indicating the best-ventilated lung areas;
2. Silent spaces, representing lung areas with minimal or no ventilation (impedance changes <10%);
3. Relative stretch, reflecting the potential of the lungs to expand during inspiration;
4. Relative tidal volume, expressed as a fraction of total ventilated lung area;
5. Distribution of tidal volume between the right and left lungs separately, referring to the sum of tidal volumes per lung and the regional distribution (ventral, dorsal, central-ventral, and central-dorsal segments).
Statistical analyses
The results were analyzed using IBM SPSS Statistics v. 22 (IBM Corp., Armonk, USA). The Mann–Whitney test was used to compare results between groups. Data from 3 episodes were compared using the Friedman test, with the Wilcoxon rank-sum test used for paired data when the Friedman’s test indicated significant differences between groups. Statistical significance was determined at p < 0.05. During analysis, 2 neonates were excluded in episode I, 1 neonate in episode II and 5 neonates in episode III due to discontinuation of EIT recording or recording errors, which precluded comparison across all 3 episodes.
Results
The 1st data recording was performed as soon as possible after birth, depending on the condition of the newborn, but no later than within 30 min (average 13 min). The 2nd recording occurred at an average of 62 min, and the 3rd one at 1.5 h (average 93 min) after birth.
Silent spaces
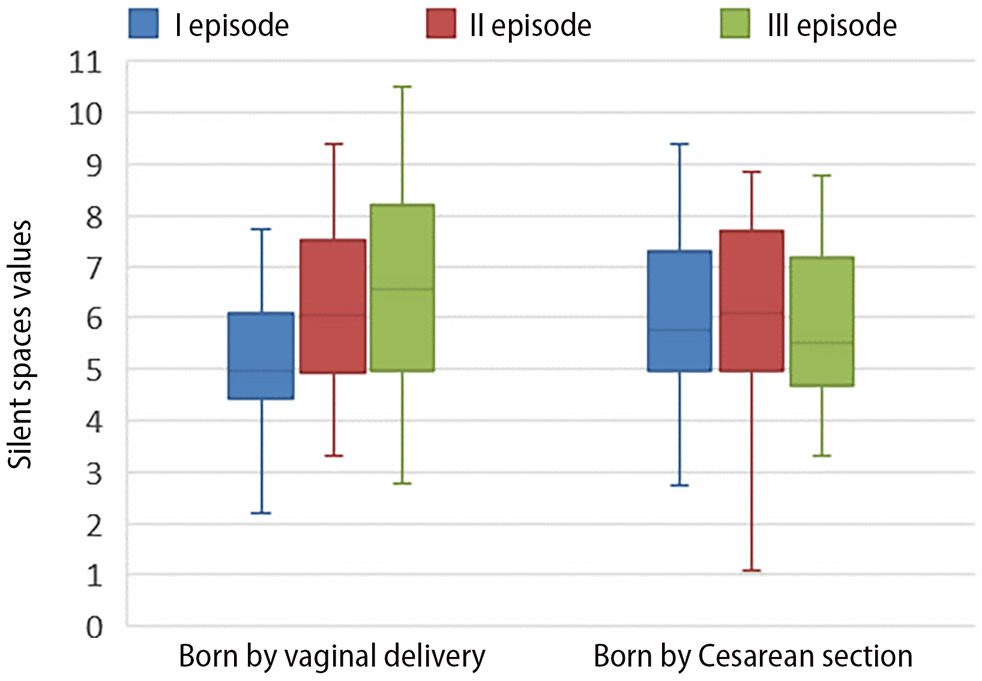
Statistically significant changes related to this parameter were observed only in the lung’s dependent regions (portions most influenced by gravity). Neonates born via CS had more silent spaces in these regions at 1 h after birth compared to those born via NVD (Table 2). The median silent space in dependent lung regions was 0 and did not differ between groups or over time, indicating that 50% of neonates had no unventilated areas in these regions (Figure 2).
Relative stretch of lung tissue
A statistically significant difference between neonates born via NVD and those born via CS was observed at 1 h and 1.5 h after birth. In the NVD group, greater stretch-related changes were observed, particularly in the least ventilated spaces, corresponding to the 25% quartile (the lowest stretch, also referred to as ‘grey spaces’). There was little to no difference in median relative stretch across episodes between neonatal groups. Lung tissue stretch induced by the same tidal volume (25%, 50% and 75%, respectively) decreased insignificantly over time in the study group and slightly increased during the last episode in the control group (Table 3).
Relative lung stretch categories of 10–20%, 20–30% and 30–40% correlated more with greater lung capacities in the study group of neonates compared to the control group (i.e., smaller changes in stretch – grey spaces occupied a larger proportion of the lung image in the study group). These changes were statistically significant at 1 h after birth (episode II) (Table 4).
Center of ventilation
The results showed no statistically significant shift in the center of ventilation between or within groups. In the study group, more neonates had a CoV value not exceeding the mean at 90 min after birth (right-to-left shift). In the ventral-dorsal projection, the variation of the center of ventilation in the study group was similar at 1 h and 1.5 h after birth.
Distribution of tidal volume
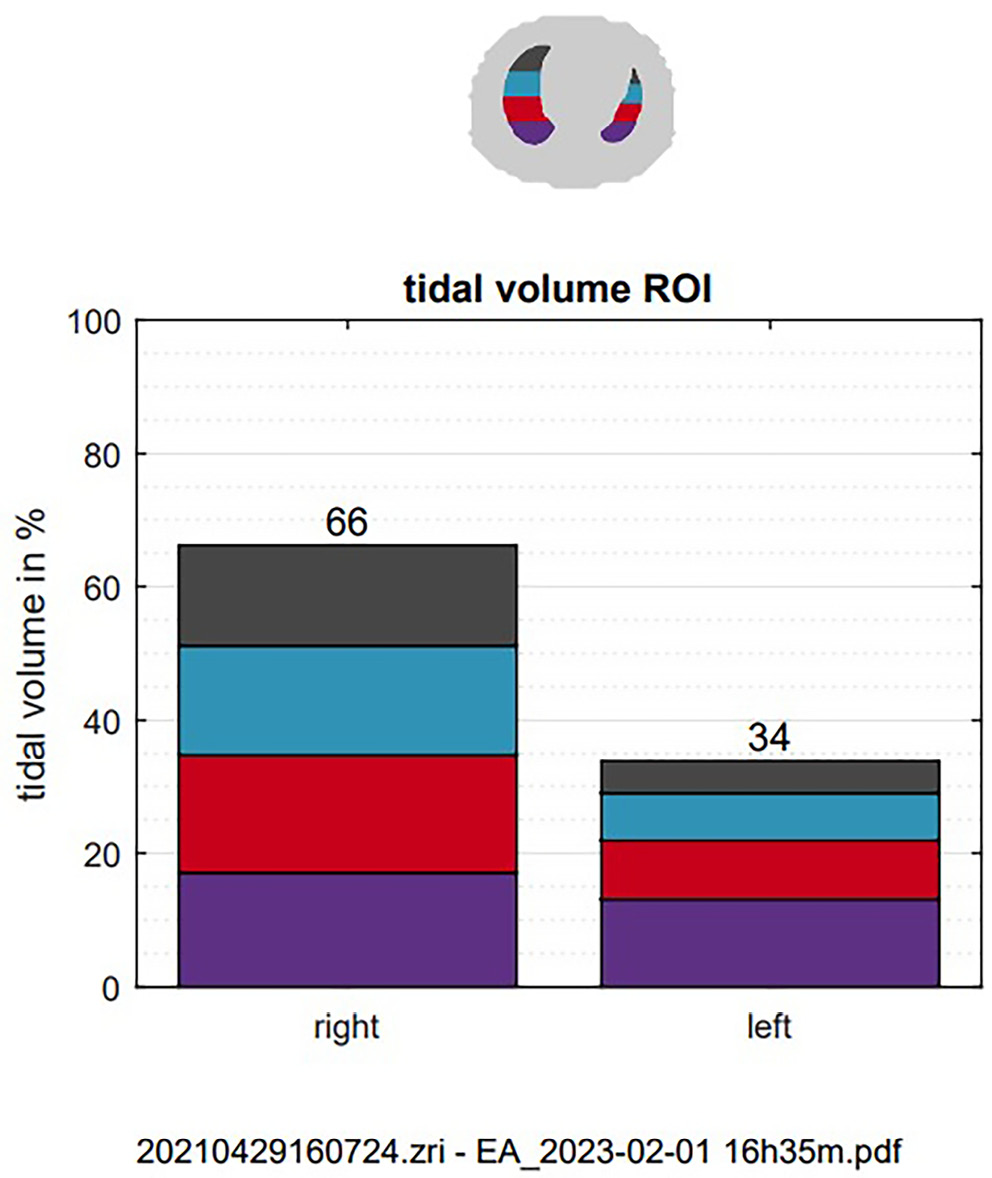
There was no significant temporal variation in the distribution of tidal volume between the left and right lung in the study and control cohorts. However, a greater distribution of tidal volume toward the central regions was observed in the right lung of the study group immediately after birth and at the end of the recording (Table 5). Neonates born via NVD had a greater volume distribution toward the central-ventral regions of the right lung immediately after birth (Table 6). Figure 3 illustrates the distribution of tidal volume between the left and right lung of a spontaneously breathing term neonate.
Discussion
There are few studies on respiratory adaptation in neonates immediately after birth, primarily due to the lack of appropriate instruments or techniques for noninvasive assessment of lung aeration in real time. Our study intended to fill this gap.
Our analysis revealed differences between the 2 study groups in several EIT parameters: silent spaces, relative stretch, relative tidal volume, and distribution of lung aeration. The most pronounced differences in aeration were observed at 1 h after birth rather than immediately post-birth. Neonates born via NVD had significantly fewer unventilated (or poorly ventilated) spaces in the dependent lung regions. This aligns with prior research indicating that in healthy neonates without mechanical ventilation, dependent lung regions (those most affected by gravity) exhibit better aeration compared to independent lung regions (those least influenced by gravity).9 Conversely, a higher distribution of aeration to independent lung regions was generally found in intensive care unit (ICU) patients and was associated with worse respiratory status.10 However, Schinckel et al. concluded that gravity plays a limited role in stable, spontaneously breathing neonates, and that ventilation patterns are more anatomically determined.11 Larger silent spaces in neonates born by CS may be due to retained pulmonary fluid or alveolar collapse caused by a lack of surfactant. According to a study by Blank et al., comparing lung adaptation between identical groups of neonates using ultrasound, complete lung fluid clearance after birth can take several hours, and differences between groups may persist beyond 1.5 h.12 We observed statistically significant differences in ventilation in several EIT parameters in episode II, but these differences persisted only in 1 parameter – relative stretch – in episode III. In episode II, neonates born by elective CS showed the highest percentage of relative tidal volume in both lungs (grey spaces on EIT images), primarily in areas with the lowest stretch (10–20%, 20–30% and 30–40%). These spaces correspond to areas with reduced aeration, although they receive slightly more air than silent spaces, where impedance changes are below 10%. Lung tissue expansion depends on the proportion of tidal volume entering the lung, and is, therefore, the highest in well-ventilated areas. Finally, changes in the distribution of aeration were identified only in neonates born by normal vaginal delivery, and these changes varied over time. This suggests that central ventral lung areas received the most air immediately after birth, with ventilation becoming more homogeneous by the end of the study.
Previous studies have associated CS births with higher rates of respiratory distress syndrome, transient tachypnea of the newborn and persistent pulmonary hypertension of the newborn.13, 14, 15 In normal vaginal deliveries, maternal catecholamines are believed to determine the regulation of surfactant production and sodium ion transport across epithelial cells, facilitating lung fluid resorption. During the transition period, the increase in pulmonary perfusion must be consistent with an increase in aeration; otherwise, the normal adaptation of the newborn is impaired. However, because neonates born by CS have slower lung fluid clearance and greater amounts of residual lung fluid, optimal gas exchange is achieved over a longer period. In addition, there is less surfactant produced to cover the alveolar surface.16 If CS is performed before spontaneous labor onset, neonatal lungs are significantly less mature than in the case of post-labor CS.17, 18, 19
Post-CS respiratory changes during the early adaptation period have been described in animal studies using contrast-enhanced lung X-ray imaging. However, the invasiveness and radiation risks limit its clinical utility.20 A 2018 study using lung ultrasound compared neonates born by different modes of delivery but found significant differences only at baseline, which disappeared within 2 h after birth. Regardless of delivery mode, all neonates achieved lung aeration and partial fluid resorption within 20 min after birth, with complete fluid clearance occurring within several hours.12
To date, numerous publications have focused on short- and long-term respiratory morbidity associated with the mode of delivery.21, 22, 23, 24, 25 However, there are few publications describing differences in lung adaptation among term neonates in the first hours after birth. In 2017, Bhargava et al. analyzed differences in oxygen saturation between term neonates delivered vaginally and via CS and found significantly lower saturation levels in the CS group during the initial 30 min after birth. Neonates typically take 12–14 min to reach a saturation rate of 95%. These findings are consistent with our observation that adaptation to extrauterine life is slower in the CS group.26
The first breaths after birth establish the lung functional residual capacity (FRC), increase pulmonary circulation and facilitate carbon dioxide (CO2) removal. Expired CO2 levels (ECO2) correlate with lung aeration and indicate airway patency and pulmonary gas exchange. However, measurements of ECO2, respiratory rate and tidal volume in neonates delivered vaginally vs by CS did not reveal significant differences in lung adaptation during the transition period. These measurements were performed during the first 30 min of life.27 In contrast, Finn et al. reported a delay in the establishment of FRC in the CS group. This is consistent with our study and with previous plethysmography findings,28 suggesting that larger amounts of fluid in the lung interstitium may delay the time for gas exchange to become optimal in neonatal lungs.29 However, in the study by Finn et al.,28 this delay was most notable in the initial minutes of life, whereas in our EIT study, it became apparent at the end of the first hour of life.
Blank et al. used lung ultrasound to monitor changes in lung aeration in healthy neonates and found that partial lung fluid resorption occurred within the first 20 min after birth, with full FRC achieved within 4 h. Neonates born by different modes of delivery were compared using a validated scoring system: higher scores indicated better lung aeration and fluid clearance. Neonates delivered by elective CS had significantly lower lung aeration at 1–10 min and 11–20 min after birth compared to those born vaginally and those delivered by emergency CS. According to Blank et al., neonates born by elective CS had insignificantly lower lung aeration at 1 h after birth compared to other groups, whereas our EIT study recorded the most significant difference between the study and control groups at 1 h after birth. Lung ultrasound showed no difference between the NVD and CS groups. Significantly better aeration was observed via echocardiography in neonates delivered by emergency CS compared to those in the elective CS group (up to 1 h after birth).30 In term pregnancies, such surgeries are usually performed due to intrauterine hypoxia or failed vaginal delivery, often after the onset of labor and subsequent lung fluid clearance. Thus, it poses less risk for respiratory adaptation compared to elective CS performed before the onset of spontaneous labor when there is incomplete lung maturation and surfactant deficiency. A more detailed EIT study is needed in the future to compare these CS groups separately.
Martelius et al. used ultrasound to study postnatal lungs in term infants. They observed a significant decrease in the abundance of B-lines, indicative of lung fluid migration, during the first 24 h in both groups of infants born by normal vaginal delivery and elective CS. At 1 h after delivery, there was no significant difference between the groups, but by 3 h after birth, CS was associated with significantly higher lung fluid content than normal vaginal delivery. However, this difference disappeared at 24 h after birth.31, 32 Compared with lung ultrasound, EIT identified delayed fluid clearance and poorer aeration earlier in neonates born by elective CS.
Limitations
The limitations of our study include the fact that neonates were only examined in the supine position; they were not placed in the prone position. Another limitation is that the study was conducted at a single center.
Conclusions
No statistically significant differences in lung aeration immediately after birth were found between neonates born by normal vaginal delivery and neonates born by CS. At 1 h after birth, poorer ventilation was observed in the CS group, particularly in dependent lung regions and in both lungs combined. After 1.5 h, more pronounced changes related to lung tissue stretch persisted in the NVD group. In this group, the tidal volume distribution steadily increased throughout the 1.5-h observation period. Electrical impedance tomography is a suitable method for assessing lung aeration in term neonates during early adaptation.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.